Effects of cannabinoid receptor agonists on neuronally-evoked contractions of urinary bladder tissues isolated from rat, mouse, pig, dog, monkey and human
- PMID: 10780977
- PMCID: PMC1571997
- DOI: 10.1038/sj.bjp.0703229
Effects of cannabinoid receptor agonists on neuronally-evoked contractions of urinary bladder tissues isolated from rat, mouse, pig, dog, monkey and human
Abstract
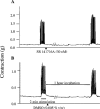
This study investigated the cannabinoid receptor, known to inhibit neuronally-evoked contractions of the mouse isolated urinary bladder, in bladder sections isolated from mouse, rat, dog, pig non-human primate or human. The CB(1)-like pharmacology of the cannabinoid receptor in mouse isolated bladder observed previously was confirmed in this study by the rank order of agonist potencies: CP 55940>/=WIN 55212-2>HU 210>JWH 015>anandamide, the high affinity of the CB(1) selective antagonist, SR 141716A (apparent pK(B) 8.7), and the low affinity of the CB(2) antagonist, SR 144528 (apparent pK(B)<6.5). In these studies, SR 141716A (10-100 nM) significantly potentiated electrically-evoked contractions in this tissue by an undetermined mechanism. A similar rank order of agonist potencies was determined in rat isolated bladder sections (CP 55, 940> or =WIN 55212-2>JWH 015). In this tissue, the maximal inhibitory effect of all agonists was lower than in the mouse bladder. Indeed, the effects of both HU 210 and anandamide were too modest to quantify potency accurately. In the rat isolated bladder, SR 141716A (30 nM) or SR 144528 (100 nM), reversed the inhibitory effect of WIN 55212-2 (apparent pK(B) = 8.4 and 8.0, respectively) or JWH 015 (apparent pK(B) = 8.2 and 7.4, respectively). These findings may demonstrate pharmacological differences between the rat and mouse orthologues of the CB(1) receptor. Alternatively, they may be attributed to a mixed population of CB(1) and CB(2) receptors that jointly influence neurogenic contraction of the rat bladder, but cannot be differentiated without more selective ligands. WIN 55212-2 had no effect on electrically-evoked contractions of bladder sections isolated from dog, pig, cynomolgus monkey and human. These findings suggest that the effect of cannabinoid agonists to inhibit neurogenic contraction of the mouse and rat bladder is not conserved across all mammalian species.
Figures
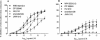
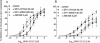
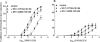
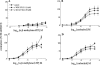


References
-
- ANDERSON K.E. Pharmacology of the lower urinary tract smooth muscles and penile erectile tissues. Pharmacol. Rev. 1993;45:253–308. - PubMed
-
- BREIVOGEL C.S., SIM L.J., CHILDERS S.R. Regional differences in cannabinoid receptor/G-protein coupling in rat brain. J. Pharmacol. Exp. Ther. 1997;282:1632–1642. - PubMed
-
- GABELLA G. Intramural neurons in the urinary bladder of the guinea-pig. Cell Tissue Res. 1990;261:231–237. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

