Comparison of the activity and disposition of the novel cholesterol absorption inhibitor, SCH58235, and its glucuronide, SCH60663
- PMID: 10780982
- PMCID: PMC1571998
- DOI: 10.1038/sj.bjp.0703235
Comparison of the activity and disposition of the novel cholesterol absorption inhibitor, SCH58235, and its glucuronide, SCH60663
Abstract
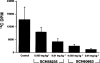
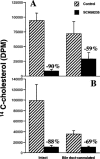
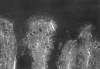
Previous studies described the metabolism-based discovery of a potent, selective inhibitor of intestinal absorption of cholesterol, SCH58235 (Ezetimibe). Here we demonstrate that the phenolic glucuronide (SCH60663) of SCH58235, was more potent at inhibiting cholesterol absorption in rats than SCH58235, when administered by the intraduodenal route. To understand the increased potency of the glucuronide, the metabolism and distribution of SCH58235 and SCH60663 were studied in bile duct-cannulated rats. One minute after intraduodenal delivery of SCH58235, significant levels of compound were detected in portal plasma; >95% was glucuronidated, indicating that the intestine was metabolizing SCH58235 to its glucuronide. When intraduodenally delivered as SCH58235, the compound was glucuronidated, moved through the intestinal wall, into portal plasma, through the liver, and into bile. However, when delivered as SCH60663, >95% of the compound remained in the intestinal lumen and wall, which may explain its increased potency. Significant inhibition of cholesterol absorption and glucuronidation of SCH58235 occurred when SCH58235 was intravenously injected into bile duct-cannulated rats. Autoradiographic analysis demonstrated that drug related material was located throughout the intestinal villi, but concentrated in the villus tip. These data indicate that (a) SCH58235 is rapidly metabolized in the intestine to its glucuronide; (b) once glucuronidated, the dose is excreted in the bile, thereby delivering drug related material back to the site of action and (c) the glucuronide is more potent than the parent possibly because it localizes to the intestine. Taken together, these data may explain the potency of SCH58235 in the rat (ID(50) = 0.0015 mg kg(-1)) and rhesus monkey (ID(50) = 0.0005 mg kg(-1)).
Figures




References
-
- BACK D.J., ROGERS S.M. Review: first-pass metabolism by the gastrointestinal mucosa. Aliment. Pharmacol. Ther. 1987;5:339–357. - PubMed
-
- BERGMAN M., MORALES H., MELLARS L., KOSOGLOU T., BURRIER R., DAVIS H.R., Jr, SYBERTZ E.J., POLLARE T.The clinical development of a novel cholesterol absorption inhibitor XII International Symposium on Drugs Affecting Lipid Metabolism 1995(abstract)
-
- BURNETT D.A., CAPLEN M.A., DAVIS H.R., BURRIER R.E., CLADER J.W. 2-Azetidinones as inhibitors of cholesterol absorption. J. Med. Chem. 1994;37:1733–1736. - PubMed
-
- DAVIS H.R., WATKINS R.W., SALISBURY B.G., COMPTON D.S., SYBERTZ E.J., BURRIER R.E. Effect of the cholesterol absorption inhibitor SCH48461 in combination with the HMG-CoA reductase inhibitor lovastatin in rabbits, dogs, and rhesus monkeys. Atherosclerosis. 1994;109:162.
-
- DAVIS H.R., VAN HEEK M., WATKINS R.W., ROSENBLUM S.B., COMPTON D.S., HOOS L., MCGREGOR D.G., PULA K., SYBERTZ E.J. The hypocholesterolemic activity of the potent cholesterol absorption inhibitor SCH58235 alone and in combination with HMG CoA reductase inhibitors (abstract) XII International Symposium on Drugs Affecting Lipid Metabolism (DALM) 1995.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

