Evolution of the bilaterian body plan: what have we learned from annelids?
- PMID: 10781038
- PMCID: PMC34316
- DOI: 10.1073/pnas.97.9.4434
Evolution of the bilaterian body plan: what have we learned from annelids?
Abstract
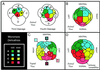
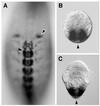
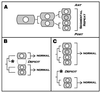
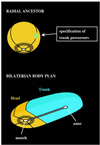
Annelids, unlike their vertebrate or fruit fly cousins, are a bilaterian taxon often overlooked when addressing the question of body plan evolution. However, recent data suggest that annelids offer unique insights on the early evolution of spiral cleavage, anteroposterior axis formation, body axis segmentation, and head versus trunk distinction.
Figures
References
-
- Brusca R C, Brusca G J. Invertebrates. Sunderland, MA: Sinauer; 1990.
-
- Wilson E B. J Morphol. 1892;6:361–480.
-
- Adoutte A, Balavoine G, Lartillot N, de Rosa R. Trends Genet. 1999;15:104–108. - PubMed
-
- Henry J J, Martindale M Q. Dev Biol. 1998;201:253–269. - PubMed
-
- Boyer B C, Henry J J, Martindale M Q. Dev Biol. 1998;204:111–123. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

