Robust perfect adaptation in bacterial chemotaxis through integral feedback control
- PMID: 10781070
- PMCID: PMC18287
- DOI: 10.1073/pnas.97.9.4649
Robust perfect adaptation in bacterial chemotaxis through integral feedback control
Abstract
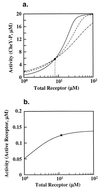
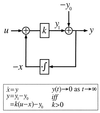
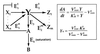
Integral feedback control is a basic engineering strategy for ensuring that the output of a system robustly tracks its desired value independent of noise or variations in system parameters. In biological systems, it is common for the response to an extracellular stimulus to return to its prestimulus value even in the continued presence of the signal-a process termed adaptation or desensitization. Barkai, Alon, Surette, and Leibler have provided both theoretical and experimental evidence that the precision of adaptation in bacterial chemotaxis is robust to dramatic changes in the levels and kinetic rate constants of the constituent proteins in this signaling network [Alon, U., Surette, M. G., Barkai, N. & Leibler, S. (1998) Nature (London) 397, 168-171]. Here we propose that the robustness of perfect adaptation is the result of this system possessing the property of integral feedback control. Using techniques from control and dynamical systems theory, we demonstrate that integral control is structurally inherent in the Barkai-Leibler model and identify and characterize the key assumptions of the model. Most importantly, we argue that integral control in some form is necessary for a robust implementation of perfect adaptation. More generally, integral control may underlie the robustness of many homeostatic mechanisms.
Figures
Comment in
-
Cell signaling pathways as control modules: complexity for simplicity?Proc Natl Acad Sci U S A. 2000 May 9;97(10):5031-3. doi: 10.1073/pnas.97.10.5031. Proc Natl Acad Sci U S A. 2000. PMID: 10805765 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

