Outer mitochondrial membrane permeability can regulate coupled respiration and cell survival
- PMID: 10781072
- PMCID: PMC18290
- DOI: 10.1073/pnas.090082297
Outer mitochondrial membrane permeability can regulate coupled respiration and cell survival
Abstract
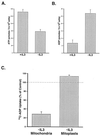
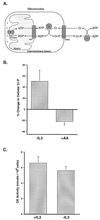
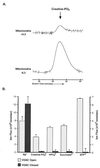
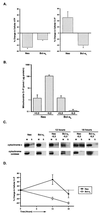
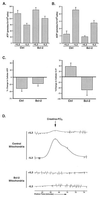
Coupled cellular respiration requires that ATP and ADP be efficiently exchanged between the cytosol and the mitochondrial matrix. When growth factors are withdrawn from dependent cells, metabolism is disrupted by a defect in ATP/ADP exchange across the mitochondrial membranes. Unexpectedly, we find that this defect results from loss of outer mitochondrial membrane permeability to metabolic anions. This decrease in anion permeability correlates with the changes in conductance properties that accompany closure of the voltage-dependent anion channel (also known as mitochondrial porin). Loss of outer membrane permeability (i) results in the accumulation of stored metabolic energy within the intermembrane space in the form of creatine phosphate, (ii) is prevented by the outer mitochondrial membrane proteins Bcl-x(L) and Bcl-2, and (iii) can be reversed by growth factor readdition. If outer membrane impermeability persists, the disruption of mitochondrial homeostasis culminates in loss of outer mitochondrial membrane integrity, cytochrome c redistribution, and apoptosis. The recognition that outer membrane permeability is regulated under physiological conditions has important implications for the understanding of bioenergetics and cell survival.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

