D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor
- PMID: 10781100
- PMCID: PMC18334
- DOI: 10.1073/pnas.97.9.4926
D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor
Abstract
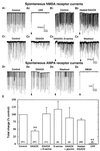
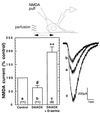
Functional activity of N-methyl-D-aspartate (NMDA) receptors requires both glutamate binding and the binding of an endogenous coagonist that has been presumed to be glycine, although D-serine is a more potent agonist. Localizations of D-serine and it biosynthetic enzyme serine racemase approximate the distribution of NMDA receptors more closely than glycine. We now show that selective degradation of d-serine with D-amino acid oxidase greatly attenuates NMDA receptor-mediated neurotransmission as assessed by using whole-cell patch-clamp recordings or indirectly by using biochemical assays of the sequelae of NMDA receptor-mediated calcium flux. The inhibitory effects of the enzyme are fully reversed by exogenously applied D-serine, which by itself did not potentiate NMDA receptor-mediated synaptic responses. Thus, D-serine is an endogenous modulator of the glycine site of NMDA receptors and fully occupies this site at some functional synapses.
Figures



References
-
- Hashimoto A, Oka T. Prog Neurobiol. 1997;52:325–353. - PubMed
-
- Dunlop D S, Neidle A, McHale D, Dunlop D M, Lajtha A. Biochem Biophys Res Commun. 1986;141:27–32. - PubMed
-
- Hashimoto A, Nishikawa T, Oka T, Hayashi T, Takahashi K. FEBS Lett. 1993;331:4–8. - PubMed
-
- Hashimoto A, Nishikawa T, Oka T, Takahashi K. J Neurochem. 1993;60:783–786. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

