The leucine-rich repeat protein SUR-8 enhances MAP kinase activation and forms a complex with Ras and Raf
- PMID: 10783161
- PMCID: PMC316541
The leucine-rich repeat protein SUR-8 enhances MAP kinase activation and forms a complex with Ras and Raf
Abstract
Caenorhabditis elegans sur-8 encodes a positive regulator of Ras signaling. We investigated the mechanism by which the human Sur-8 homolog can positively regulate Ras-MAP kinase signaling in mammalian cells. Sur-8 expression enhances Ras- or EGF-induced Raf and ERK activation but has no effect on ERK activation induced by active Raf or MEK. Furthermore, Sur-8 expression does not increase AKT or JNK activation. Sur-8 interacts with Ras and Raf and is able to form a ternary complex with the two proteins. Thus, Sur-8 may function as a scaffold that enhances Ras-MAP kinase signal transduction by facilitating the interaction between Ras and Raf.
Figures







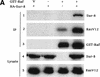




References
-
- Chang HC, Karim FD, O'Neill EM, Rebay I, Solomon NM, Therrien M, Wassarman DA, Wolff T, Rubin GM. Ras signal transduction pathway in Drosophila eye development. Cold Spring Harb Symp Quant Biol. 1994;59:147–153. - PubMed
-
- Derijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: A protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. - PubMed
-
- Field J, Xu HP, Michaeli T, Ballester R, Sass P, Wigler M, Colicelli J. Mutations of the adenylyl cyclase gene that block RAS function in Saccharomyces cerevisiae. Science. 1990;247:464–467. - PubMed
-
- Guan KL, Dixon JE. Eukaryotic proteins expressed in Escherichia coli: An improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991;192:262–267. - PubMed
-
- Howe LR, Leevers SJ, Gomez N, Nakielny S, Cohen P, Marshall CJ. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992;71:335–342. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
