Proteolysis in Caenorhabditis elegans sex determination: cleavage of TRA-2A by TRA-3
- PMID: 10783162
- PMCID: PMC316540
Proteolysis in Caenorhabditis elegans sex determination: cleavage of TRA-2A by TRA-3
Abstract
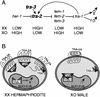
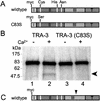
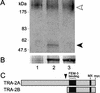
The Caenorhabditis elegans tra-3 gene promotes female development in XX hermaphrodites and encodes an atypical calpain regulatory protease lacking calcium-binding EF hands. We report that despite the absence of EF hands, TRA-3 has calcium-dependent proteolytic activity and its proteolytic domain is essential for in vivo function. We show that the membrane protein TRA-2A, which promotes XX female development by repressing the masculinizing protein FEM-3, is a TRA-3 substrate. Cleavage of TRA-2A by TRA-3 generates a peptide predicted to have feminizing activity. These results indicate that proteolysis regulated by calcium may control some aspects of sexual cell fate in C. elegans.
Figures

Similar articles
-
Germ-line regulation of the Caenorhabditis elegans sex-determining gene tra-2.Dev Biol. 1998 Dec 1;204(1):251-62. doi: 10.1006/dbio.1998.9062. Dev Biol. 1998. PMID: 9851857
-
The TRA-1 transcription factor binds TRA-2 to regulate sexual fates in Caenorhabditis elegans.EMBO J. 2001 Mar 15;20(6):1363-72. doi: 10.1093/emboj/20.6.1363. EMBO J. 2001. PMID: 11250902 Free PMC article.
-
The PLZF-like protein TRA-4 cooperates with the Gli-like transcription factor TRA-1 to promote female development in C. elegans.Dev Cell. 2006 Oct;11(4):561-73. doi: 10.1016/j.devcel.2006.07.015. Dev Cell. 2006. PMID: 17011494
-
It ain't over till it's ova: germline sex determination in C. elegans.Bioessays. 2001 Jul;23(7):596-604. doi: 10.1002/bies.1085. Bioessays. 2001. PMID: 11462213 Review.
-
Sex determination in the Caenorhabditis elegans germ line.Curr Top Dev Biol. 2008;83:41-64. doi: 10.1016/S0070-2153(08)00402-X. Curr Top Dev Biol. 2008. PMID: 19118663 Review.
Cited by
-
Identification of BiP as a temperature sensor mediating temperature-induced germline sex reversal in C. elegans.EMBO J. 2024 Sep;43(18):4020-4048. doi: 10.1038/s44318-024-00197-z. Epub 2024 Aug 12. EMBO J. 2024. PMID: 39134659 Free PMC article.
-
A Caenorhabditis elegans wild type defies the temperature-size rule owing to a single nucleotide polymorphism in tra-3.PLoS Genet. 2007 Mar 2;3(3):e34. doi: 10.1371/journal.pgen.0030034. Epub 2007 Jan 9. PLoS Genet. 2007. PMID: 17335351 Free PMC article.
-
Sex Determination in Nematode Germ Cells.Sex Dev. 2022;16(5-6):305-322. doi: 10.1159/000520872. Epub 2022 Feb 16. Sex Dev. 2022. PMID: 35172320 Free PMC article. Review.
-
Abnormal glucose homeostasis and pancreatic islet function in mice with inactivation of the Fem1b gene.Mol Cell Biol. 2005 Aug;25(15):6570-7. doi: 10.1128/MCB.25.15.6570-6577.2005. Mol Cell Biol. 2005. PMID: 16024793 Free PMC article.
-
Outcrossing and the maintenance of males within C. elegans populations.J Hered. 2010 Mar-Apr;101 Suppl 1(Suppl 1):S62-74. doi: 10.1093/jhered/esq003. Epub 2010 Mar 8. J Hered. 2010. PMID: 20212008 Free PMC article.
References
-
- Aza-Blanc P, Kornberg TB. Ci: A complex transducer of the hedgehog signal. Trends Genet. 1999;15:458–462. - PubMed
-
- Blanchard H, Grochulski P, Li Y, Arthur JS, Davies PL, Elce JS, Cygler M. Structure of a calpain Ca2+-binding domain reveals a novel EF-hand and Ca2+-induced conformational changes. Nat Struct Biol. 1997;4:532–538. - PubMed
-
- The C. elegans Sequencing Consortium (Consortium) Genome sequence of the nematode C. elegans: A platform for investigating biology. Science. 1998;282:2012–2018. - PubMed
-
- Dear N, Matena K, Vingron M, Boehm T. A new subfamily of vertebrate calpains lacking a calmodulin-like domain: Implications for calpain regulation and evolution. Genomics. 1997;45:175–184. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
