Analysis of the thyrotropin-releasing hormone-degrading ectoenzyme by site-directed mutagenesis of cysteine residues. Cys68 is involved in disulfide-linked dimerization
- PMID: 10785382
- PMCID: PMC7163949
- DOI: 10.1046/j.1432-1327.2000.01277.x
Analysis of the thyrotropin-releasing hormone-degrading ectoenzyme by site-directed mutagenesis of cysteine residues. Cys68 is involved in disulfide-linked dimerization
Abstract
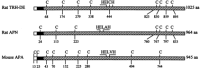
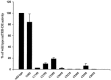
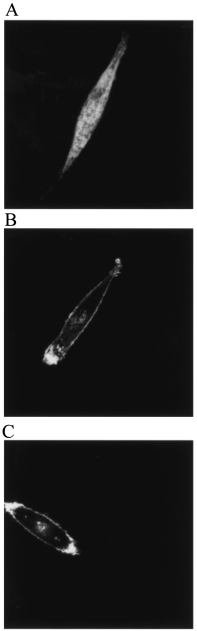
Thyrotropin-releasing hormone-degrading ectoenzyme is a member of the M1 family of Zn-dependent aminopeptidases and catalyzes the degradation of thyrotropin-releasing hormone (TRH; Glp-His-Pro-NH2). Cloning of the cDNA of this enzyme and biochemical studies revealed that the large extracellular domain of the enzyme with the catalytically active site contains nine cysteine residues that are highly conserved among species. To investigate the functional role of these cysteines in TRH-DE we used a site-directed mutagenesis approach and replaced individually each cysteine by a serine residue. The results revealed that the proteolytically truncated and enzymatically fully active enzyme consists of two identical subunits that are associated noncovalently by protein-protein interactions but not via interchain S-S bridges. The eight cysteines contained within this region are all important for the structure of the individual subunit and the enzymatic activity, which is dramatically reduced in all mutant enzymes. This is even true for the four cysteines that are clustered within the C-terminal domain remote from the Zn-binding consensus sequence HEICH. In contrast, Cys68, which resides within the stalk region seven residues from the end of the hydrophobic membrane-spanning domain, can be replaced by serine without a significant change in the enzymatic activity. Interestingly, this residue is involved in the formation of an interchain disulfide bridge. Covalent dimerization of the subunits, however, does not seem to be essential for efficient biosynthesis, enzymatic activity and trafficking to the cell surface.
Figures




Similar articles
-
Mutational analysis of the thyrotropin-releasing hormone-degrading ectoenzyme. similarities and differences with other members of the M1 family of aminopeptidases and thermolysin.Biochemistry. 2001 Aug 7;40(31):9347-55. doi: 10.1021/bi010695w. Biochemistry. 2001. PMID: 11478903
-
A disulfide bonding interaction role for cysteines in the extracellular domain of the thyrotropin-releasing hormone receptor.Endocrinology. 1996 Jul;137(7):2851-8. doi: 10.1210/endo.137.7.8770906. Endocrinology. 1996. PMID: 8770906
-
Human TRH-degrading ectoenzyme cDNA cloning, functional expression, genomic structure and chromosomal assignment.Eur J Biochem. 1999 Oct 1;265(1):415-22. doi: 10.1046/j.1432-1327.1999.00753.x. Eur J Biochem. 1999. PMID: 10491199
-
The thyrotropin-releasing hormone-degrading ectoenzyme: the third element of the thyrotropin-releasing hormone-signaling system.Thyroid. 1998 Oct;8(10):915-20. doi: 10.1089/thy.1998.8.915. Thyroid. 1998. PMID: 9827659 Review.
-
Thyrotropin releasing hormone (TRH), the TRH-receptor and the TRH-degrading ectoenzyme; three elements of a peptidergic signalling system.Results Probl Cell Differ. 1999;26:13-42. doi: 10.1007/978-3-540-49421-8_2. Results Probl Cell Differ. 1999. PMID: 10453458 Review. No abstract available.
Cited by
-
Mutation of the membrane-associated M1 protease APM1 results in distinct embryonic and seedling developmental defects in Arabidopsis.Plant Cell. 2009 Jun;21(6):1693-721. doi: 10.1105/tpc.108.059634. Epub 2009 Jun 16. Plant Cell. 2009. PMID: 19531600 Free PMC article.
-
The C-terminal domain, but not the interchain disulphide, is required for the activity and intracellular trafficking of aminopeptidase A.Biochem J. 2002 Feb 15;362(Pt 1):191-7. doi: 10.1042/0264-6021:3620191. Biochem J. 2002. PMID: 11829756 Free PMC article.
-
Extracellular cysteines define ectopeptidase (APN, CD13) expression and function.Free Radic Biol Med. 2002 Apr 1;32(7):584-95. doi: 10.1016/s0891-5849(01)00827-9. Free Radic Biol Med. 2002. PMID: 11909693 Free PMC article.
References
-
- Guillemin, R. (1978) Peptides in the brain: the new endocrinology of the neuron. Science 202, 390–302. - PubMed
-
- Schally, A.V. (1978) Aspects of hypothalamic regulation of the pituirary gland. Science 202, 18–28. - PubMed
-
- Morley, J.E. (1981) Neuroendocrine control of thyrotropin secretion. Endocr. Rev. 2, 396–436. - PubMed
-
- Prasad, C. (1984) Thyrotropin‐releasing hormone In Handbook of Neurochemistry ( Lajtha A., ed.), Vol. 8, pp. 175–200. Plenum, New York.
-
- Kelly, J.A. (1995) Thyrotropin releasing hormone: basis and potential for its therapeutic use. Essays Biochem. 30, 133–149. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

