Detection and identification of Leishmania DNA within naturally infected sand flies by seminested PCR on minicircle kinetoplastic DNA
- PMID: 10788363
- PMCID: PMC101436
- DOI: 10.1128/AEM.66.5.1933-1938.2000
Detection and identification of Leishmania DNA within naturally infected sand flies by seminested PCR on minicircle kinetoplastic DNA
Abstract
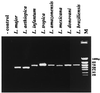
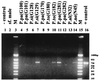
A seminested PCR assay was developed in order to amplify the kinetoplast minicircle of Leishmania species from individual sand flies. The kinetoplast minicircle is an ideal target because it is present in 10,000 copies per cell and its sequence is known for most Leishmania species. The two-step PCR is carried out in a single tube using three primers, which were designed within the conserved area of the minicircle and contain conserved sequence blocks. The assay was able to detect as few as 3 parasites per individual sand fly and to amplify minicircle DNA from at least eight Leishmania species. This technique permits the processing of a large number of samples synchronously, as required for epidemiological studies, in order to study infection rates in sand fly populations and to identify potential insect vectors. Comparison of the sequences obtained from sand flies and mammal hosts will be crucial for developing hypotheses about the transmission cycles of Leishmania spp. in areas of endemicity.
Figures

References
-
- Adini I, Jacobson R L, Kasap M, Schlein Y, Jaffe C L. Species-specific detection of Leishmania in sandflies using an enzyme-linked immunosorbent assay. Trans R Soc Trop Med Hyg. 1998;92:35–37. - PubMed
-
- Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
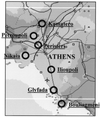
- Aransay A M, Scoulica E, Chaniotis B, Tselentis Y. Typing of sandflies from Greece and Cyprus by DNA polymorphism of 18S rRNA gene. Insect Mol Biol. 1999;8:179–184. - PubMed
-
- Brewster S, Aslett M, Barker D C. Kinetoplast DNA minicircle database. Parasitol Today. 1998;14:437–438. - PubMed
-
- de Bruijn M H, Barker D C. Diagnosis of New World leishmaniasis: specific detection of species of the Leishmania braziliensis complex by amplification of kinetoplast DNA. Acta Trop. 1992;52:45–58. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

