Redox chemistry in laccase-catalyzed oxidation of N-hydroxy compounds
- PMID: 10788380
- PMCID: PMC101453
- DOI: 10.1128/AEM.66.5.2052-2056.2000
Redox chemistry in laccase-catalyzed oxidation of N-hydroxy compounds
Abstract
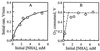
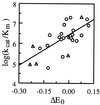
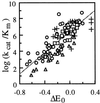
1-Hydroxybenzotriazole, violuric acid, and N-hydroxyacetanilide are three N-OH compounds capable of mediating a range of laccase-catalyzed biotransformations, such as paper pulp delignification and degradation of polycyclic hydrocarbons. The mechanism of their enzymatic oxidation was studied with seven fungal laccases. The oxidation had a bell-shaped pH-activity profile with an optimal pH ranging from 4 to 7. The oxidation rate was found to be dependent on the redox potential difference between the N-OH substrate and laccase. A laccase with a higher redox potential or an N-OH compound with a lower redox potential tended to have a higher oxidation rate. Similar to the enzymatic oxidation of phenols, phenoxazines, phenothiazines, and other redox-active compounds, an "outer-sphere" type of single-electron transfer from the substrate to laccase and proton release are speculated to be involved in the rate-limiting step for N-OH oxidation.
Figures


References
-
- Amitai G, Adani R, Sod-Moriah G, Rabinovitz I, Vincze A, Leader H, Chefetz B, Leibovitz-Persky L, Friesem D, Hadar Y. Oxidative biodegradation of phosphorothiolates by laccase. FEBS Lett. 1998;438:195–200. - PubMed
-
- Ander P, Messner K. Oxidation of 1-hydroxybenzotriazole by laccase and lignin peroxidase. Biotechnol Techniques. 1998;12:191–195.
-
- Andrieux C P, Saveant J-M. Homogeneous redox catalysis of electrochemical reactions: electron transfers followed by a very fast chemical step. J Electroanal Chem. 1986;205:43–58.
-
- Aurich H G, Bach G, Hahn K, Küttner G, Weiss W. Aminyloxide (Nitroxide). XXV. Reaktionen von Benzotriazolyloxid-radikalen mit Aromaten. J Chem Res. 1977;1997:1537–1545.
-
- Berka R M, Schneider P, Golightly E J, Brown S H, Madden M, Brown K M, Halkier T, Mondorf K, Xu F. Characterization of the gene encoding an extracellular polyphenoloxidase of Myceliophthora thermophila and analysis of the recombinant enzyme expressed in Aspergillus oryzae. Appl Environ Microbiol. 1997;63:3151–3157. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

