Nitrite reductase genes (nirK and nirS) as functional markers to investigate diversity of denitrifying bacteria in pacific northwest marine sediment communities
- PMID: 10788387
- PMCID: PMC101460
- DOI: 10.1128/AEM.66.5.2096-2104.2000
Nitrite reductase genes (nirK and nirS) as functional markers to investigate diversity of denitrifying bacteria in pacific northwest marine sediment communities
Abstract
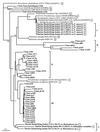
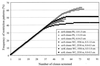
Genetic heterogeneity of denitrifying bacteria in sediment samples from Puget Sound and two sites on the Washington continental margin was studied by PCR approaches amplifying nirK and nirS genes. These structurally different but functionally equivalent single-copy genes coding for nitrite reductases, a key enzyme of the denitrification process, were used as a molecular marker for denitrifying bacteria. nirS sequences could be amplified from samples of both sampling sites, whereas nirK sequences were detected only in samples from the Washington margin. To assess the underlying nir gene structure, PCR products of both genes were cloned and screened by restriction fragment length polymorphism (RFLP). Rarefraction analysis revealed a high level of diversity especially for nirS clones from Puget Sound and a slightly lower level of diversity for nirK and nirS clones from the Washington margin. One group dominated within nirK clones, but no dominance and only a few redundant clones were seen between sediment samples for nirS clones in both habitats. Hybridization and sequencing confirmed that all but one of the 228 putative nirS clones were nirS with levels of nucleotide identities as low as 45.3%. Phylogenetic analysis grouped nirS clones into three distinct subclusters within the nirS gene tree which corresponded to the two habitats from which they were obtained. These sequences had little relationship to any strain with known nirS sequences or to isolates (mostly close relatives of Pseudomonas stutzeri) from the Washington margin sediment samples. nirK clones were more closely related to each other than were the nirS clones, with 78.6% and higher nucleotide identities; clones showing only weak hybridization signals were not related to known nirK sequences. All nirK clones were also grouped into a distinct cluster which could not be placed with any strain with known nirK sequences. These findings show a very high diversity of nir sequences within small samples and that these novel nir clusters, some very divergent from known sequences, are not known in cultivated denitrifiers.
Figures


References
-
- Ausubel F M, Brent R, Kingston E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1991.
-
- Berks B C, Moir J W B, Richardson D J. Enzymes and associated electron transport systems that catalyse the respiratory reduction of nitrogen oxides and oxyanions. Biochim Biophys Acta. 1995;1232:97–173. - PubMed
-
- Carpenter R, Peterson M L, Bennett J T. 210Pb-derived sediment accumulation and mixing rates for the Washington continental slope. Mar Chem. 1982;48:135–164.
-
- Codispoti L A. Is the ocean losing nitrate? Nature. 1995;376:724.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

