Detection of and discrimination between gram-positive and gram-negative bacteria in intraocular samples by using nested PCR
- PMID: 10790093
- PMCID: PMC86579
- DOI: 10.1128/JCM.38.5.1753-1757.2000
Detection of and discrimination between gram-positive and gram-negative bacteria in intraocular samples by using nested PCR
Abstract
A nested PCR protocol has been developed for the detection of and discrimination between 14 species of gram-positive and -negative bacteria in samples of ocular fluids. First-round PCR with pan-bacterial oligonucleotide primers, based on conserved sequences of the 16S ribosomal gene, was followed by a gram-negative-organism-specific PCR, which resulted in a single 985-bp amplification product, and a multiplex PCR which resulted in two PCR products: a 1,025 bp amplicon (all bacteria) and a 355 bp amplicon (gram-positive bacteria only). All products were detected by gel electrophoresis. The sensitivity of the assay was between 10 fg and 1 pg of bacterial DNA, depending on the species tested, equivalent to between 24 and 4 live bacteria spiked in water. The identification was complete in 3.5 h. The molecular techniques were subsequently applied to four samples of intraocular fluid, (three vitreous and one aqueous) from three patients with clinical signs of bacterial endophthalmitis (test samples) and two samples of vitreous from a patient with chronic intraocular inflammation (control samples). In all culture-positive samples (two of three vitreous and one of one aqueous), a complete concordance was observed between molecular methods and culture results. PCR correctly identified the gram stain classification of the organisms. The bacterial etiology was also identified in a culture-negative patient with clinical history and signs highly suggestive of bacterial endophthalmitis. Furthermore, control samples from a patient with chronic intraocular inflammation remained PCR negative. In summary, this protocol has demonstrated potential as a rapid diagnostic test in confirming the diagnosis of infection and also determining the Gram status of bacteria with high specificity and sensitivity.
Figures
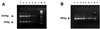

References
-
- Bazra M, Pavan P R, Doft B H, Wisniewski S R, Wilson L A, Han D P, Kelsey S F. Evaluation of microbiological diagnostic techniques in postoperative endophthalmitis in the endophthalmitis vitrectomy study. Arch Ophthalmol. 1997;115:1142–1150. - PubMed
-
- Bej A K, Mahbubani M H, Miller R, DiCesare J L, Haff L, Atlas R M. Multiplex PCR amplification and immobilized capture probes for the detection of bacterial pathogens and indicators in water. Mol Cell Probes. 1990;4:353–365. - PubMed
-
- Bohigian G M, Olk R J. Factors associated with a poor visual result in endophthalmitis. Am J Ophthalmol. 1986;101:332–341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

