MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fiber type
- PMID: 10790363
- PMCID: PMC305686
- DOI: 10.1093/emboj/19.9.1963
MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fiber type
Abstract
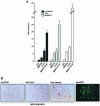
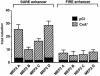
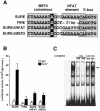
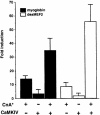
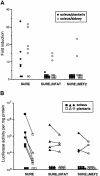
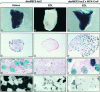
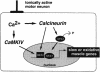
Different patterns of motor nerve activity drive distinctive programs of gene transcription in skeletal muscles, thereby establishing a high degree of metabolic and physiological specialization among myofiber subtypes. Recently, we proposed that the influence of motor nerve activity on skeletal muscle fiber type is transduced to the relevant genes by calcineurin, which controls the functional activity of NFAT (nuclear family of activated T cell) proteins. Here we demonstrate that calcineurin-dependent gene regulation in skeletal myocytes is mediated also by MEF2 transcription factors, and is integrated with additional calcium-regulated signaling inputs, specifically calmodulin-dependent protein kinase activity. In skeletal muscles of transgenic mice, both NFAT and MEF2 binding sites are necessary for properly regulated function of a slow fiber-specific enhancer, and either forced expression of activated calcineurin or motor nerve stimulation up-regulates a MEF2-dependent reporter gene. These results provide new insights into the molecular mechanisms by which specialized characteristics of skeletal myofiber subtypes are established and maintained.
Figures



References
-
- Bito H., Deisseroth,K. and Tsien,R.W. (1996) CREB phosphorylation and dephosphorylation: a Ca2+- and stimulus duration-dependent switch for hippocampal gene expression. Cell, 87, 1203–1214. - PubMed
-
- Black B.L. and Olson,E.N. (1998) Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu. Rev. Cell Dev. Biol., 14, 167–196. - PubMed
-
- Black B.L., Martin,J.F. and Olson,E.N. (1995) The mouse MRF4 promoter is trans-activated directly and indirectly by muscle-specific transcription factors. J. Biol. Chem., 270, 2889–2892. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

