The Id4 HLH protein and the timing of oligodendrocyte differentiation
- PMID: 10790366
- PMCID: PMC305683
- DOI: 10.1093/emboj/19.9.1998
The Id4 HLH protein and the timing of oligodendrocyte differentiation
Abstract
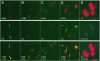
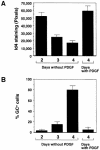
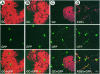
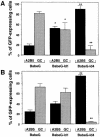
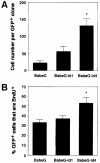
An intracellular timer is thought to help control the timing of oligodendrocyte differentiation. We show here that the expression of the helix-loop-helix gene Id4 in oligodendrocyte precursor cells decreases in vivo and in vitro with a time course expected if Id4 is part of the timer. We also show that Id4 expression decreases prematurely when the precursor cells are induced to differentiate by mitogen withdrawal. Both Id4 mRNA and protein decrease together under all of these conditions, suggesting that the control of Id4 expression is transcriptional. Finally, we show that enforced expression of Id4 stimulates cell proliferation and blocks differentiation induced by either mitogen withdrawal or treatment with thyroid hormone. These findings suggest that a progressive fall in Id4 transcription is part of the intracellular timer that helps determine when oligodendrocyte precursor cells withdraw from the cell cycle and differentiate.
Figures




References
-
- Ahlgren S.C., Wallace,H., Bishop,J., Neophytou,C. and Raff,M. (1997) Effects of thyroid hormone on embryonic oligodendrocyte precursor cell development in vivo and in vitro. Mol. Cell. Neurosci., 9, 420–432. - PubMed
-
- Ausubel F.M., Brent,R., Kingston,R.E., Moore,D.D., Seidman,J.G., Smith,J.A. and Struhl,K. (1992) Current Protocols in Molecular Biology. Wiley, New York, NY, pp. 9.10.1–9.14.3.
-
- Barres B. and Raff,M. (1994) Control of oligodendrocyte number in the developing rat optic nerve. Neuron, 12, 935–942. - PubMed
-
- Barres B., Hart,I., Coles,H., Burne,J., Voyvodic,J., Richardson,W. and Raff,M. (1992) Cell death and control of cell survival in the oligodendrocyte lineage. Cell, 70, 31–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

