Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication
- PMID: 10790373
- PMCID: PMC305685
- DOI: 10.1093/emboj/19.9.2069
Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication
Abstract
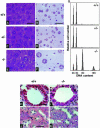
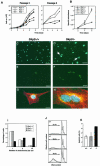
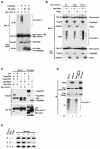
The ubiquitin-proteasome pathway plays an important role in control of the abundance of cell cycle regulators. Mice lacking Skp2, an F-box protein and substrate recognition component of an Skp1-Cullin-F-box protein (SCF) ubiquitin ligase, were generated. Although Skp2(-/-) animals are viable, cells in the mutant mice contain markedly enlarged nuclei with polyploidy and multiple centrosomes, and show a reduced growth rate and increased apoptosis. Skp2(-/-) cells also exhibit increased accumulation of both cyclin E and p27(Kip1). The elimination of cyclin E during S and G(2) phases is impaired in Skp2(-/-) cells, resulting in loss of cyclin E periodicity. Biochemical studies showed that Skp2 interacts specifically with cyclin E and thereby promotes its ubiquitylation and degradation both in vivo and in vitro. These results suggest that specific degradation of cyclin E and p27(Kip1) is mediated by the SCF(Skp2) ubiquitin ligase complex, and that Skp2 may control chromosome replication and centrosome duplication by determining the abundance of cell cycle regulators.
Figures



References
-
- Bai C., Sen,P., Hofmann,K., Ma,L., Goebl,M., Harper,J.W. and Elledge,S.J. (1996) SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell, 86, 263–274. - PubMed
-
- Carrano A.C., Eytan,E., Hershko,A. and Pagano,M. (1999) SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nature Cell Biol., 1, 193–199. - PubMed
-
- Clurman B.E., Sheaff,R.J., Thress,K., Groudine,M. and Roberts,J.M. (1996) Turnover of cyclin E by the ubiquitin–proteasome pathway is regulated by cdk2 binding and cyclin phosphorylation. Genes Dev., 10, 1979–1990. - PubMed
-
- Correa-Bordes J. and Nurse,P. (1995) p25Rum1 orders S phase and mitosis by acting as an inhibitor of the p34cdc2 mitotic kinase. Cell, 83, 1001–1009. - PubMed
-
- Dealy M., Nguyen,K.V.T., Lo,J., Gstaiger,M., Krek,W., Elson,D., Arbeit,J., Kipreos,E.T. and Johnson,R.S. (1999) Loss of Cul1 results in early embryonic lethality and dysregulation of cyclin E. Nature Genet., 23, 245–248. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

