Rcl1p, the yeast protein similar to the RNA 3'-phosphate cyclase, associates with U3 snoRNP and is required for 18S rRNA biogenesis
- PMID: 10790377
- PMCID: PMC305690
- DOI: 10.1093/emboj/19.9.2115
Rcl1p, the yeast protein similar to the RNA 3'-phosphate cyclase, associates with U3 snoRNP and is required for 18S rRNA biogenesis
Abstract
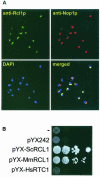
RNA 3'-terminal phosphate cyclases are evolutionarily conserved enzymes catalysing conversion of the 3'-terminal phosphate in RNA to the 2',3'-cyclic phosphodiester. Their biological role remains unknown. The yeast Saccharomyces cerevisiae contains a gene encoding a protein with strong sequence similarity to the characterized cyclases from humans and Escherichia coli. The gene, named RCL1 (for RNA terminal phosphate cyclase like), is essential for growth, and its product, Rcl1p, is localized in the nucleolus. Depletion or inactivation of Rcl1p impairs pre-rRNA processing at sites A(0), A(1) and A(2), and leads to a strong decrease in 18S rRNA and 40S ribosomal subunit levels. Immunoprecipitations indicate that Rcl1p is specifically associated with the U3 snoRNP, although, based on gradient analyses, it is not its structural component. Most of Rcl1p sediments in association with the 70-80S pre-ribosomal particle and a 10S complex of unknown identity. Proteins similar to Rcl1p are encoded in genomes of all eukaryotes investigated and the mouse orthologue complements yeast strains depleted of Rcl1p. Possible functions of Rcl1p in pre-rRNA processing and its relationship to the RNA 3'-phosphate cyclase are discussed.
Figures







References
-
- Arn E.A. and Abelson,J.N. (1998) RNA ligases: function, mechanism and sequence conservation. In Simons,R.W. and Grunberg-Manago,M. (eds), RNA Structure and Function. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 695–726.
-
- Balakin A.G., Smith,L. and Fournier,M.J. (1996) The RNA world of the nucleolus: two major families of small RNAs defined by different box elements with related functions. Cell, 86, 823–834. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

