The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a protective antigen and a major virulence factor
- PMID: 10790422
- PMCID: PMC2213429
- DOI: 10.1084/jem.191.9.1467
The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a protective antigen and a major virulence factor
Abstract
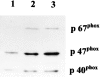
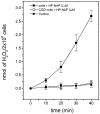
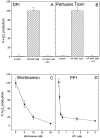
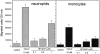
Helicobacter pylori infection induces the appearance of inflammatory infiltrates, consisting mainly of neutrophils and monocytes, in the human gastric mucosa. A bacterial protein with neutrophil activating activity (HP-NAP) has been previously identified, but its role in infection and immune response is still largely unknown. Here, we show that vaccination of mice with HP-NAP induces protection against H. pylori challenge, and that the majority of infected patients produce antibodies specific for HP-NAP, suggesting an important role of this factor in immunity. We also show that HP-NAP is chemotactic for human leukocytes and that it activates their NADPH oxidase to produce reactive oxygen intermediates, as demonstrated by the translocation of its cytosolic subunits to the plasma membrane, and by the lack of activity on chronic granulomatous disease leukocytes. This stimulating effect is strongly potentiated by tumor necrosis factor alpha and interferon gamma and is mediated by a rapid increase of the cytosolic calcium concentration. The activation of leukocytes induced by HP-NAP is completely inhibited by pertussis toxin, wortmannin, and PP1. On the basis of these results, we conclude that HP-NAP is a virulence factor important for the H. pylori pathogenic effects at the site of infection and a candidate antigen for vaccine development.
Figures

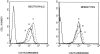



Comment in
-
Modulating phagocyte activation: the pros and cons of Helicobacter pylori virulence factors.J Exp Med. 2000 May 1;191(9):1451-4. doi: 10.1084/jem.191.9.1451. J Exp Med. 2000. PMID: 10790419 Free PMC article. No abstract available.
References
-
- Warren J.D., Marshall B.J. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273–1275. - PubMed
-
- Marshall B.J., Armstrong J.A., McGechie D.B., Glancy R.J. Attempt to fulfill Koch's postulates for pyloric Campylobacter . Med. J. Austr. 1985;142:436–439. - PubMed
-
- Goodwin C.S. Helicobacter pylori gastritis, peptic ulcer and gastric cancerclinical and molecular aspects. Clin. Infect. Dis. 1997;25:1017–1019. - PubMed
-
- Bayerdorffer E., Lehn N., Hatz R., Mannes G.A., Oertel H., Sauerbruch T., Stolte M. Difference in expression of Helicobacter pylori gastritis in antrum and body. Gastroenterology. 1992;102:1575–1582. - PubMed
-
- Fiocca R., Villani L., Luinetti O., Gianotti A., Perego M., Alvisi C., Turpini F., Solcia E. Helicobacter colonization and histopathological profile of chronic gastritis in patients with or without dyspepsia, mucosal erosion and peptic ulcera morphological approach to the study of ulcerogenesis in man. Virchows Archiv. A Pathol. Anat. Histopathol. 1992;420:489–492. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

