Differential regulation of B cell development, activation, and death by the src homology 2 domain-containing 5' inositol phosphatase (SHIP)
- PMID: 10790429
- PMCID: PMC2213431
- DOI: 10.1084/jem.191.9.1545
Differential regulation of B cell development, activation, and death by the src homology 2 domain-containing 5' inositol phosphatase (SHIP)
Abstract
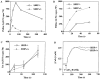
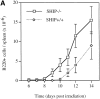
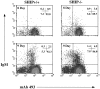
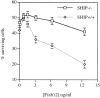
Although the Src homology 2 domain-containing 5' inositol phosphatase (SHIP) is a well-known mediator of inhibitory signals after B cell antigen receptor (BCR) coaggregation with the low affinity Fc receptor, it is not known whether SHIP functions to inhibit signals after stimulation through the BCR alone. Here, we show using gene-ablated mice that SHIP is a crucial regulator of BCR-mediated signaling, B cell activation, and B cell development. We demonstrate a critical role for SHIP in termination of phosphatidylinositol 3,4,5-triphosphate (PI[3,4,5]P(3)) signals that follow BCR aggregation. Consistent with enhanced PI(3,4,5)P(3) signaling, we find that splenic B cells from SHIP-deficient mice display enhanced sensitivity to BCR-mediated induction of the activation markers CD86 and CD69. We further demonstrate that SHIP regulates the rate of B cell development in the bone marrow and spleen, as B cell precursors from SHIP-deficient mice progress more rapidly through the immature and transitional developmental stages. Finally, we observe that SHIP-deficient B cells have increased resistance to BCR-mediated cell death. These results demonstrate a central role for SHIP in regulation of BCR signaling and B cell biology, from signal driven development in the bone marrow and spleen, to activation and death in the periphery.
Figures





References
-
- Benschop R.J., Cambier J.C. B cell developmentsignal transduction by antigen receptors and their surrogates. Curr. Opin. Immunol. 1999;11:143–151. - PubMed
-
- Lam K.P., Kuhn R., Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–1083. - PubMed
-
- Kitamura D., Roes J., Kuhn R., Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. - PubMed
-
- Kitamura D., Kudo A., Schaal S., Muller W., Melchers F., Rajewsky K. A critical role of lambda 5 protein in B cell development. Cell. 1992;69:823–831. - PubMed
-
- Gong S., Nussenzweig M.C. Regulation of an early developmental checkpoint in the B cell pathway by Ig beta. Science. 1996;272:411–414. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

