The removal of human breast cancer cells from hematopoietic CD34+ stem cells by dielectrophoretic field-flow-fractionation
- PMID: 10791899
- PMCID: PMC2726259
- DOI: 10.1089/152581699319939
The removal of human breast cancer cells from hematopoietic CD34+ stem cells by dielectrophoretic field-flow-fractionation
Abstract
Dielectrophoretic field-flow-fractionation (DEP-FFF) was used to purge human breast cancer MDA-435 cells from hematopoietic CD34+ stem cells. An array of interdigitated microelectrodes lining the bottom surface of a thin chamber was used to generate dielectrophoretic forces that levitated the cell mixture in a fluid flow profile. CD34+ stem cells were levitated higher, were carried faster by the fluid flow, and exited the separation chamber earlier than the cancer cells. Using on-line flow cytometry, efficient separation of the cell mixture was observed in less than 12 min, and CD34+ stem cell fractions with a purity >99.2% were obtained. The method of DEP-FFF is potentially applicable to many biomedical cell separation problems, including microfluidic-scale diagnosis and preparative-scale purification of cell subpopulations.
Figures



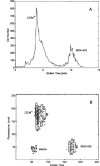
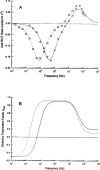
Comment in
-
Ex vivo purging and cell expansion.J Hematother Stem Cell Res. 1999 Oct;8(5):457-8. doi: 10.1089/152581699319902. J Hematother Stem Cell Res. 1999. PMID: 10791896 No abstract available.
References
-
- Gribben JG, Freedman AS, Neuberg D, Roy DC, Blake KW, Woo SD, Grossbard ML, Rabinowe SN, Coral F, et al. Immunologic purging of marrow assessed by PCR before autologous bone marrow transplantation for B-cell lymphoma. N Engl J Med. 1991;325:1525–1533. - PubMed
-
- Collins RH., Jr. CD34+ selected cells in clinical transplantation. Stem Cells. 1993;12:577–585. - PubMed
-
- Ross AA, Coope BW, Lazarus HM, et al. Detection and viability of tumor cells in peripheral blood stem cell collections from breast cancer patients using immunocytochemical and clonogenic assay techniques. Blood. 1993;82:2605–2610. - PubMed
-
- Rummel SA, VanZant G. Future paradigm for autologous bone marrow transplantation: tumor purging and ex vivo production of normal stem and progenitor cells. J Hematother. 1994;3:213–218. - PubMed
-
- Santos GW. The role of autologous hematopoietic stem cell transplantation in hematologic malignancy. Curr Opin Oncol. 1994;6:115–121. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
