Calcineurin activity is required for the initiation of skeletal muscle differentiation
- PMID: 10791979
- PMCID: PMC2174840
- DOI: 10.1083/jcb.149.3.657
Calcineurin activity is required for the initiation of skeletal muscle differentiation
Abstract
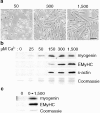
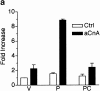
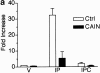
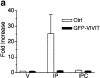
Differentiation of skeletal muscle myoblasts follows an ordered sequence of events: commitment, cell cycle withdrawal, phenotypic differentiation, and finally cell fusion to form multinucleated myotubes. The molecular signaling pathways that regulate the progression are not well understood. Here we investigate the potential role of calcium and the calcium-dependent phosphatase calcineurin in myogenesis. Commitment, phenotypic differentiation, and cell fusion are identified as distinct calcium-regulated steps, based on the extracellular calcium concentration required for the expression of morphological and biochemical markers specific to each of these stages. Furthermore, differentiation is inhibited at the commitment stage by either treatment with the calcineurin inhibitor cyclosporine A (CSA) or expression of CAIN, a physiological inhibitor of calcineurin. Retroviral-mediated gene transfer of a constitutively active form of calcineurin is able to induce myogenesis only in the presence of extracellular calcium, suggesting that multiple calcium-dependent pathways are required for differentiation. The mechanism by which calcineurin initiates differentiation includes transcriptional activation of myogenin, but does not require the participation of NFAT. We conclude that commitment of skeletal muscle cells to differentiation is calcium and calcineurin-dependent, but NFAT-independent.
Figures








References
-
- Aramburu J., Yaffe M.B., Lopez-Rodriguez C., Cantley L.C., Hogan P.G., Rao A. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science. 1999;285:2129–2133. - PubMed
-
- Barnoy S., Glaser T., Kosower N.S. Calpain and calpastatin in myoblast differentiation and fusioneffects of inhibitors. Biochim. Biophys. Acta. 1997;1358:181–188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

