Matrix survival signaling: from fibronectin via focal adhesion kinase to c-Jun NH(2)-terminal kinase
- PMID: 10791986
- PMCID: PMC2174844
- DOI: 10.1083/jcb.149.3.741
Matrix survival signaling: from fibronectin via focal adhesion kinase to c-Jun NH(2)-terminal kinase
Abstract
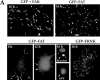
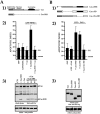
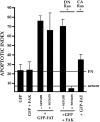
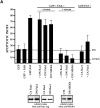
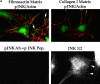
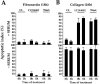
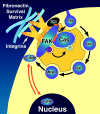
Most transformed cells have lost anchorage and serum dependence for growth and survival. Previously, we established that when serum is absent, fibronectin survival signals transduced by focal adhesion kinase (FAK), suppress p53-regulated apoptosis in primary fibroblasts and endothelial cells (Ilić et al. 1998. J. Cell Biol. 143:547-560). The present goals are to identify survival sequences in FAK and signaling molecules downstream of FAK required for anchorage-dependent survival of primary fibroblasts. We report that binding of the SH3 domain of p130Cas to proline-rich region 1 of FAK is required to support survival of fibroblasts on fibronectin when serum is withdrawn. The FAK-p130Cas complex activates c-Jun NH2-terminal kinase (JNK) via a Ras/Rac1/Pak1/MAPK kinase 4 (MKK4) pathway. Activated (phospho-) JNK colocalizes with FAK in focal adhesions of fibroblasts cultured on fibronectin, which supports their survival, but not in fibroblasts cultured on collagen, which does not. Cells often survive in the absence of extracellular matrix if serum factors are provided. In that case, we confirm work of others that survival signals are transduced by FAK, phosphatidylinositol 3'-kinase (PI3-kinase), and Akt/protein kinase B (PKB). However, when serum is absent, PI3-kinase and Akt/PKB are not involved in the fibronectin-FAK-JNK survival pathway documented herein. Thus, survival signals from extracellular matrix and serum are transduced by FAK via two distinct pathways.
Figures








References
-
- Anderson S.M., Reyland M.E., Hunter S., Deisher L.M., Barzen K.A., Quissell D.O. Etoposide-induced activation of c-jun N-terminal kinase (JNK) correlates with drug-induced apoptosis in salivary gland acinar cells. Cell Death Differ. 1999;6:454–462. - PubMed
-
- Cardone M.H., Salvesen G.S., Widmann C., Johnson G., Frisch S.M. The regulation of anoikisMEKK-1 activation requires cleavage by caspases. Cell. 1997;90:315–323. - PubMed
-
- Chaudhary P.M., Eby M.T., Jasmin A., Hood L. Activation of the c-Jun N-terminal kinase/stress-activated protein kinase pathway by overexpression of caspase-8 and its homologs. J. Biol. Chem. 1999;274:19211–19219. - PubMed
-
- Chen H.C., Appeddu P.A., Isoda H., Guan J.L. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J. Biol. Chem. 1996;271:26329–26334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

