Pathophysiological role of leptin in obesity-related hypertension
- PMID: 10791999
- PMCID: PMC315441
- DOI: 10.1172/JCI8341
Pathophysiological role of leptin in obesity-related hypertension
Abstract
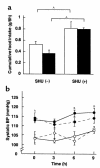
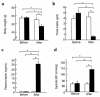
To explore the pathophysiological role of leptin in obesity-related hypertension, we examined cardiovascular phenotypes of transgenic skinny mice whose elevated plasma leptin concentrations are comparable to those seen in obese subjects. We also studied genetically obese KKA(y) mice with hyperleptinemia, in which hypothalamic melanocortin system is antagonized by ectopic expression of the agouti protein. Systolic blood pressure (BP) and urinary catecholamine excretion are elevated in transgenic skinny mice relative to nontransgenic littermates. The BP elevation in transgenic skinny mice is abolished by alpha(1)-adrenergic, beta-adrenergic, or ganglionic blockers at doses that do not affect BP in nontransgenic littermates. Central administration of an alpha-melanocyte-stimulating hormone antagonist causes a marked increase in cumulative food intake but no significant changes in BP. The obese KKA(y) mice develop BP elevation with increased urinary catecholamine excretion relative to control KK mice. After a 2-week caloric restriction, BP elevation is reversed in nontransgenic littermates with the A(y) allele, in parallel with a reduction in plasma leptin concentrations, but is sustained in transgenic mice overexpressing leptin with the A(y) allele, which remain hyperleptinemic. This study demonstrates BP elevation in transgenic skinny mice and obese KKA(y) mice that are both hyperleptinemic, thereby suggesting the pathophysiological role of leptin in some forms of obesity-related hypertension.
Figures
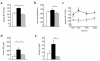

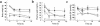
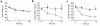
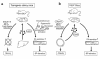
References
-
- Kannel WB, Brand M, Skinner JJ, Jr, Sawber TR, McNamara PM. The relation of adiposity to blood pressure and development of hypertension: the Framingham study. Ann Intern Med. 1967;67:48–59. - PubMed
-
- Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. - PubMed
-
- Landsberg L, Krieger DR. Obesity, metabolism, and the sympathetic nervous system. Am J Hypertension. 1989;2:125S–132S. - PubMed
-
- Mark AL, Correia M, Morgan DA, Shaffer RA, Haynes WG. Obesity-Induced Hypertension : New Concepts From the Emerging Biology of Obesity. Hypertension. 1999;33:537–541. - PubMed
-
- Rocchini AP, et al. The effect of weight loss on the sensitivity of blood pressure to sodium in obese adolescents. N Engl J Med. 1989;31:580–585. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

