M-CSF neutralization and egr-1 deficiency prevent ovariectomy-induced bone loss
- PMID: 10792003
- PMCID: PMC315442
- DOI: 10.1172/JCI8672
M-CSF neutralization and egr-1 deficiency prevent ovariectomy-induced bone loss
Abstract
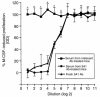
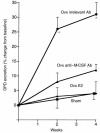
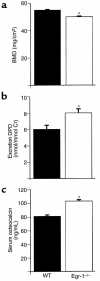
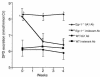
Increased stromal cell production of M-CSF, an event caused by enhanced phosphorylation of the nuclear protein Egr-1, is central to the mechanism by which estrogen (E2) deficiency upregulates osteoclast (OC) formation. However, the contribution of enhanced M-CSF production to the bone loss induced by E2 deficiency remains to be determined. We found that treatment with an Ab that neutralizes M-CSF in vivo completely prevents the rise in OC number, the increase in bone resorption, and the resulting bone loss induced by ovariectomy (ovx). We also found that adult, intact Egr-1-deficient mice, a strain characterized by maximally stimulated stromal cell production of M-CSF, exhibit increased bone resorption and decreased bone mass. In these mice, treatment with anti-M-CSF Ab restored normal levels of bone resorption, thus confirming that increased M-CSF production accounts for the remodeling abnormalities of Egr-1-deficient mice. Consistent with the failure of ovx to further increase M-CSF production in Egr-1-deficient mice, ovx neither increased bone resorption further, nor caused bone loss in these animals. In summary, the data demonstrate that E2 deficiency induces M-CSF production via an Egr-1-dependent mechanism that is central to the pathogenesis of ovx-induced bone loss. Thus, Egr-1 and M-CSF are critical mediators of the bone sparing effects of E2 in vivo.
Figures


Similar articles
-
Estrogen blocks M-CSF gene expression and osteoclast formation by regulating phosphorylation of Egr-1 and its interaction with Sp-1.J Clin Invest. 1998 Nov 15;102(10):1850-9. doi: 10.1172/JCI4561. J Clin Invest. 1998. PMID: 9819371 Free PMC article.
-
Increased production of IL-7 uncouples bone formation from bone resorption during estrogen deficiency.J Clin Invest. 2002 Dec;110(11):1643-50. doi: 10.1172/JCI15687. J Clin Invest. 2002. PMID: 12464669 Free PMC article.
-
Anti-c-fms Antibody Prevents Osteoclast Formation and Bone Resorption in Co-Culture of Osteoblasts and Osteoclast Precursors In Vitro and in Ovariectomized Mice.Int J Mol Sci. 2020 Aug 25;21(17):6120. doi: 10.3390/ijms21176120. Int J Mol Sci. 2020. PMID: 32854340 Free PMC article.
-
Effect of ormeloxifene on ovariectomy-induced bone resorption, osteoclast differentiation and apoptosis and TGF beta-3 expression.J Steroid Biochem Mol Biol. 2006 Aug;100(4-5):117-28. doi: 10.1016/j.jsbmb.2006.03.009. Epub 2006 Jun 21. J Steroid Biochem Mol Biol. 2006. PMID: 16797179
-
Estrogen and testosterone use different cellular pathways to inhibit osteoclastogenesis and bone resorption.J Bone Miner Res. 2005 Dec;20(12):2224-32. doi: 10.1359/JBMR.050803. Epub 2005 Aug 1. J Bone Miner Res. 2005. PMID: 16294275
Cited by
-
Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha.J Clin Invest. 2000 Nov;106(10):1229-37. doi: 10.1172/JCI11066. J Clin Invest. 2000. PMID: 11086024 Free PMC article.
-
Selective deletion of the membrane-bound colony stimulating factor 1 isoform leads to high bone mass but does not protect against estrogen-deficiency bone loss.J Bone Miner Metab. 2012 Jul;30(4):408-18. doi: 10.1007/s00774-011-0336-y. Epub 2011 Nov 23. J Bone Miner Metab. 2012. PMID: 22105655 Free PMC article.
-
Ovariectomy disregulates osteoblast and osteoclast formation through the T-cell receptor CD40 ligand.Proc Natl Acad Sci U S A. 2011 Jan 11;108(2):768-73. doi: 10.1073/pnas.1013492108. Epub 2010 Dec 27. Proc Natl Acad Sci U S A. 2011. PMID: 21187391 Free PMC article.
-
Molecular changes in peripheral blood involving osteoarthritic joint remodelling.J Oral Rehabil. 2019 Sep;46(9):820-827. doi: 10.1111/joor.12810. Epub 2019 May 11. J Oral Rehabil. 2019. PMID: 31046158 Free PMC article.
-
Key Triggers of Osteoclast-Related Diseases and Available Strategies for Targeted Therapies: A Review.Front Med (Lausanne). 2017 Dec 20;4:234. doi: 10.3389/fmed.2017.00234. eCollection 2017. Front Med (Lausanne). 2017. PMID: 29326938 Free PMC article. Review.
References
-
- Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. N Engl J Med. 1995;332:305–311. - PubMed
-
- Suda T, et al. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20:345–357. - PubMed
-
- Lacey DL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. - PubMed
-
- Kong YY, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

