Th1 and Th2 mediate acute graft-versus-host disease, each with distinct end-organ targets
- PMID: 10792004
- PMCID: PMC315439
- DOI: 10.1172/JCI7894
Th1 and Th2 mediate acute graft-versus-host disease, each with distinct end-organ targets
Abstract
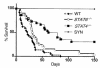
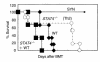
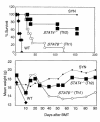
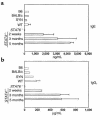
STAT4 and STAT6 are transcription factors that play crucial roles in responding to IL-12 and IL-4, respectively. STAT4 gene knockout (STAT4(-/-)) mice have markedly reduced Th1 responses and enhanced Th2 responses. STAT6(-/-) mice show the inverse phenotype. We compared the ability of bone marrow transplantation (BMT) with the inclusion of spleen cells from STAT6(-/-), STAT4(-/-), and wild-type (WT) mice to produce graft-versus-host disease (GVHD) in lethally irradiated MHC-mismatched recipients. Acute GVHD mortality was more rapid when induced by cells from STAT6(-/-) mice than when induced by STAT4(-/-) cells. However, cells from STAT4(-/-) and STAT6(-/-) donors both induced delayed GVHD mortality compared with WT controls, or compared with combined STAT4(-/-) and STAT6(-/-) cells, indicating a contribution of both Th1 cells and Th2 cells to acute GVHD. Recipients of STAT6(-/-) BMT showed evidence of acute GVHD with severe diarrhea and marked weight loss. Recipients of STAT4(-/-) BMT showed signs of GVHD with only initial transient weight loss and later development of severe skin GVHD. Histopathology showed that Th2 responses were required for the induction of both hepatic and severe skin GVHD. In contrast, both Th1 cells and Th2 cells were capable of causing intestinal pathology of GVHD. Our studies demonstrate an additive role for Th1 and Th2 cells in producing acute GVHD, and suggest a cytokine-directed approach to treating end-organ manifestations of GVHD.
Figures

Similar articles
-
Ligation of OX40 (CD134) regulates graft-versus-host disease (GVHD) and graft rejection in allogeneic bone marrow transplant recipients.Blood. 2003 May 1;101(9):3741-8. doi: 10.1182/blood-2002-10-3048. Epub 2003 Jan 9. Blood. 2003. PMID: 12521997
-
The susceptibility to experimental myasthenia gravis of STAT6-/- and STAT4-/- BALB/c mice suggests a pathogenic role of Th1 cells.J Immunol. 2004 Jan 1;172(1):97-103. doi: 10.4049/jimmunol.172.1.97. J Immunol. 2004. PMID: 14688314
-
The role of CD154-CD40 versus CD28-B7 costimulatory pathways in regulating allogeneic Th1 and Th2 responses in vivo.J Clin Invest. 2000 Jul;106(1):63-72. doi: 10.1172/JCI9586. J Clin Invest. 2000. PMID: 10880049 Free PMC article.
-
STAT4 and STAT6, their role in cellular and humoral immunity and in diverse human diseases.Int Rev Immunol. 2024;43(6):394-418. doi: 10.1080/08830185.2024.2395274. Epub 2024 Aug 26. Int Rev Immunol. 2024. PMID: 39188021 Review.
-
Stat4- and Stat6-deficient mice as models for manipulating T helper cell responses.Biochem Soc Trans. 1997 May;25(2):359-60. doi: 10.1042/bst0250359. Biochem Soc Trans. 1997. PMID: 9191117 Review. No abstract available.
Cited by
-
Unraveling the Mechanisms of Cutaneous Graft-Versus-Host Disease.Front Immunol. 2018 May 2;9:963. doi: 10.3389/fimmu.2018.00963. eCollection 2018. Front Immunol. 2018. PMID: 29770141 Free PMC article. Review.
-
T helper17 cells are sufficient but not necessary to induce acute graft-versus-host disease.Biol Blood Marrow Transplant. 2010 Feb;16(2):170-8. doi: 10.1016/j.bbmt.2009.09.023. Epub 2009 Oct 2. Biol Blood Marrow Transplant. 2010. PMID: 19804837 Free PMC article.
-
Graft rejection as a Th1-type process amenable to regulation by donor Th2-type cells through an interleukin-4/STAT6 pathway.Blood. 2008 Dec 1;112(12):4765-75. doi: 10.1182/blood-2008-05-154278. Epub 2008 Jul 14. Blood. 2008. PMID: 18625883 Free PMC article.
-
Blocking IL-21 signaling ameliorates xenogeneic GVHD induced by human lymphocytes.Blood. 2012 Jan 12;119(2):619-28. doi: 10.1182/blood-2011-07-368027. Epub 2011 Nov 10. Blood. 2012. PMID: 22077059 Free PMC article.
-
MicroRNA in T-Cell Development and T-Cell Mediated Acute Graft-Versus-Host Disease.Front Immunol. 2018 May 7;9:992. doi: 10.3389/fimmu.2018.00992. eCollection 2018. Front Immunol. 2018. PMID: 29867969 Free PMC article. Review.
References
-
- Butturini A, Gale RP. T cell depletion in bone marrow transplantation for leukemia: current results and future directions. Bone Marrow Transplant. 1988;3:185–192. - PubMed
-
- Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. - PubMed
-
- Antin JH, Ferrara JLM. Cytokine dysregulation in acute graft-versus-host disease. Blood. 1992;80:2964–2968. - PubMed
-
- Blazar BR, Korngold R, Vallera DA. Recent advances in graft-versus-host disease (GVHD) prevention. Immunol Rev. 1997;157:79–109. - PubMed
-
- Fowler DH, Kurasawa K, Smith R, Eckhaus MA, Gress RE. Donor CD4-enriched cells of Th2 cytokine phenotype regulate graft-versus-host disease without impairing allogeneic engraftment in sublethally irradiated mice. Blood. 1994;84:3540–3549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

