Myogenic stem cell function is impaired in mice lacking the forkhead/winged helix protein MNF
- PMID: 10792059
- PMCID: PMC25843
- DOI: 10.1073/pnas.100501197
Myogenic stem cell function is impaired in mice lacking the forkhead/winged helix protein MNF
Abstract
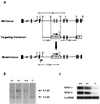
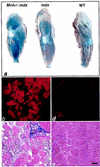
Myocyte nuclear factor (MNF) is a winged helix transcription factor that is expressed selectively in myogenic stem cells (satellite cells) of adult animals. Using a gene knockout strategy to generate a functional null allele at the Mnf locus, we observed that mice lacking MNF are viable, but severely runted. Skeletal muscles of Mnf-/- animals are atrophic, and satellite cell function is impaired. Muscle regeneration after injury is delayed and incomplete, and the normal timing of expression of cell cycle regulators and myogenic determination genes is dysregulated. Mnf mutant mice were intercrossed with mdx mice that lack dystrophin and exhibit only a subtle myopathic phenotype. In contrast, mdx mice that also lack MNF die in the first few weeks of life with a severe myopathy. Haploinsufficiency at the Mnf locus (Mnf+/-) also exacerbates the mdx phenotype to more closely resemble Duchenne's muscular dystrophy in humans. We conclude that MNF acts to regulate genes that coordinate the proliferation and differentiation of myogenic stem cells after muscle injury. Animals deficient in MNF may prove useful for evaluation of potential therapeutic interventions to promote muscle regeneration for patients having Duchenne's muscular dystrophy.
Figures

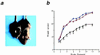



References
-
- Gussoni E, Blau H M, Kunkel L M. Nat Med. 1997;3:970–977. - PubMed
-
- Engel A G, Yamamoto M, Fischbeck K H. In: Myology. Engel A G, Franzini-Armstrong C, editors. New York: McGraw–Hill; 1994. pp. 1130–1188.
-
- Monaco A, Neve R, Colletti-Feener C, Bertelson C, Kurnit D, Kunkel L M. Nature (London) 1986;323:646–650. - PubMed
-
- Burghes A, Logan C, Hu X, Belfall B, Worton R, Ray P. Nature (London) 1987;328:434–437. - PubMed
-
- Hoffman E P, Brown R H, Kunkel L M. Cell. 1987;51:919–928. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

