Decreased CD95 expression on naive T cells from HIV-infected persons undergoing highly active anti-retroviral therapy (HAART) and the influence of IL-2 low dose administration. Irhan Study Group
- PMID: 10792383
- PMCID: PMC1905643
- DOI: 10.1046/j.1365-2249.2000.01223.x
Decreased CD95 expression on naive T cells from HIV-infected persons undergoing highly active anti-retroviral therapy (HAART) and the influence of IL-2 low dose administration. Irhan Study Group
Abstract
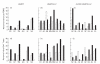
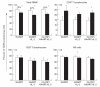
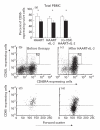
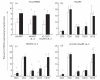
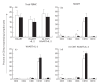
The functional recovery of the immune system in HIV-infected persons receiving HAART and the role of adjuvant immune therapy are still matters of intensive investigation. We analysed the effects of HAART combined with cytokines in 22 naive asymptomatic individuals, randomized to receive HAART (n = 6), HAART plus a low dose (1000 000 U/daily) of rIL-2 (n = 8), and HAART plus rIL-2 after previous administration of granulocyte colony-stimulating factor (n = 8). After 3 months of therapy, increased CD4+ T cell counts and diminished viral loads were observed in all patients, independently of cytokine addition. A decreased expression of CD95 (Apo 1/Fas) was evident in all groups when compared with values before therapy. The percentages of peripheral blood mononuclear cells (PBMC) expressing CD95 after therapy decreased by 15%, 22% and 18% in the three treatment groups, respectively (P < 0.05). Analysis of PBMC subsets demonstrated that CD95 expression was significantly reduced on CD45RA+CD62L+ naive T cells (25.3%, 22.4%, and 18.6%, respectively; P < 0.05) in each group, after therapy. Accordingly, all patients showed a reduced rate of in vitro spontaneous apoptosis (P < 0.05). Another effect induced by HAART was a significant increase in IL-2Ralpha expression on total PBMC (P < 0.05), independently of cytokine addition. Altogether, our results suggest that very low dose administration of rIL-2 (1000 000 U/daily) may be not enough to induce a significant improvement in the immune system as regards HAART alone. The employment of higher doses of recombinant cytokines and/or different administration protocols in clinical trials might however contribute to ameliorate the immune reconstitution in patients undergoing HAART.
Figures
References
-
- Collier AC, Coombs RW, Schoenfeld DA, et al. Treatment of human immunodeficiency virus infection with saquinavir, zidovudine and zalcitabine. N Engl J Med. 1996;334:1011–7. - PubMed
-
- Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–6. - PubMed
-
- Mathez D, Bagnarelli P, Gorin I, et al. Reduction in viral load and increases in T lymphocyte numbers in treatment-naive patients with advanced HIV-1 infection treated with ritonavir, zidovudine, zalcitabine triple therapy. Antiviral Ther. 1997;2:175–83. - PubMed
-
- Pontesilli O, Kerkhof-Garde S, Pakker NG, et al. Antigen-specific T-lymphocyte proliferative responses during highly active antiretroviral therapy (HAART) of HIV-1 infection. Immunol Letters. 1999;66:213–7. - PubMed
-
- Foudraine NA, Hovenkamp E, Notermans DW, et al. Immunopathology as a result of highly active antiretroviral therapy in HIV-1-infected patients. AIDS. 1999;13:177–84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

