Treatment of passively transferred experimental autoimmune myasthenia gravis using papain
- PMID: 10792389
- PMCID: PMC1905633
- DOI: 10.1046/j.1365-2249.2000.01202.x
Treatment of passively transferred experimental autoimmune myasthenia gravis using papain
Abstract
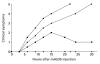
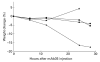
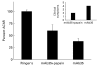
Antibody-mediated acetylcholine receptor (AChR) loss at the neuromuscular junction, the main cause of the symptoms of myasthenia gravis, is induced by bivalent or multivalent antibodies. Passive transfer of experimental autoimmune myasthenia gravis (EAMG) can be induced very efficiently in rats by administration of intact MoAbs directed against the main immunogenic region (MIR) of the AChR, but not by their monovalent Fab fragments. We tested whether papain, which has been used therapeutically in autoimmune and other diseases, is capable of preventing EAMG by in vivo cleavage of the circulating anti-AChR antibodies into Fab fragments. EAMG was induced in 4-week-old female Lewis rats by i.p. injection of anti-MIR mAb35. A total of 0.75 mg of papain was given as one or three injections 3-7 h after MoAb injection. The mAb35 + papain-treated animals developed mild weakness during the first 30 h and subsequently recovered, while all animals that received only mAb35 developed severe myasthenic symptoms and died within 24-30 h. Animals treated only with papain showed no apparent side effects for up to 2 months. Serum anti-AChR levels in mAb35 + papain-treated rats decreased within a few hours, whereas in non-papain-treated rats they remained high for at least 30 h. Muscle AChR in mAb35 + papain-treated animals was partially protected from antibody-mediated degradation. These results show that treatment of rats with papain can prevent passively transferred EAMG without any apparent harm to the animals, and suggest a potential therapeutic use for proteolytic enzymes in myasthenia gravis.
Figures


Similar articles
-
Prevention of passively transferred experimental autoimmune myasthenia gravis by Fab fragments of monoclonal antibodies directed against the main immunogenic region of the acetylcholine receptor.J Neuroimmunol. 2000 May 1;104(2):124-32. doi: 10.1016/s0165-5728(99)00259-3. J Neuroimmunol. 2000. PMID: 10713351
-
Passive transfer of experimental myasthenia gravis via antigenic modulation of acetylcholine receptor.Eur J Immunol. 1992 Sep;22(9):2449-52. doi: 10.1002/eji.1830220939. Eur J Immunol. 1992. PMID: 1516631
-
Pathological mechanisms in experimental autoimmune myasthenia gravis. II. Passive transfer of experimental autoimmune myasthenia gravis in rats with anti-acetylcholine recepotr antibodies.J Exp Med. 1976 Sep 1;144(3):739-53. doi: 10.1084/jem.144.3.739. J Exp Med. 1976. PMID: 182897 Free PMC article.
-
Acetylcholine receptor-induced experimental myasthenia gravis: what have we learned from animal models after three decades?Arch Immunol Ther Exp (Warsz). 2012 Feb;60(1):19-30. doi: 10.1007/s00005-011-0158-6. Epub 2011 Dec 8. Arch Immunol Ther Exp (Warsz). 2012. PMID: 22159475 Review.
-
Experimental Autoimmune Myasthenia Gravis (EAMG): from immunochemical characterization to therapeutic approaches.J Autoimmun. 2014 Nov;54:51-9. doi: 10.1016/j.jaut.2014.06.003. Epub 2014 Jun 24. J Autoimmun. 2014. PMID: 24970384 Review.
Cited by
-
Targeting therapy to the neuromuscular junction: proof of concept.Muscle Nerve. 2014 May;49(5):749-56. doi: 10.1002/mus.24057. Muscle Nerve. 2014. PMID: 24037951 Free PMC article.
-
Extraocular muscle characteristics related to myasthenia gravis susceptibility.PLoS One. 2013;8(2):e55611. doi: 10.1371/journal.pone.0055611. Epub 2013 Feb 8. PLoS One. 2013. PMID: 23409007 Free PMC article.
-
Guidelines for pre-clinical assessment of the acetylcholine receptor--specific passive transfer myasthenia gravis model-Recommendations for methods and experimental designs.Exp Neurol. 2015 Aug;270:3-10. doi: 10.1016/j.expneurol.2015.02.025. Epub 2015 Mar 3. Exp Neurol. 2015. PMID: 25743217 Free PMC article.
References
-
- Drachman D. Myasthenia gravis. In: Rose NR, Mackay IR, editors. The autoimmune diseases. San Diego: Academic Press; 1998. pp. 637–62.
-
- Conti-Fine BM, Protti MP, Bellone M, Howard JF. The immunobiology of an autoimmune disease. Heidelberg: Springer-Verlag; 1997. Myasthenia gravis; pp. 1–226.
-
- Massey J. Treatment of acquired myasthenia gravis. Neurology. 1997;48(Suppl. 5):S46–S51.
-
- Loutrari H, Kokla A, Tzartos SJ. Passive transfer of experimental myasthenia gravis via antigenic modulation of acetylcholine receptor. Eur J Immunol. 1992;22:2449–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

