Forced expression of terminal deoxynucleotidyl transferase in fetal thymus resulted in a decrease in gammadelta T cells and random dissemination of Vgamma3Vdelta1 T cells in skin of newborn but not adult mice
- PMID: 10792495
- PMCID: PMC2327197
- DOI: 10.1046/j.1365-2567.2000.00987.x
Forced expression of terminal deoxynucleotidyl transferase in fetal thymus resulted in a decrease in gammadelta T cells and random dissemination of Vgamma3Vdelta1 T cells in skin of newborn but not adult mice
Abstract
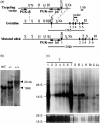
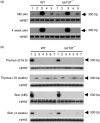
The repertoire of lymphocyte receptor genes encoded in a germline is further diversified by a number of processes, including the template-independent addition of nucleotides (N regions) by means of terminal deoxynucleotidyl transferase (TdT). Normally, mouse gammadelta T cells in the early fetal thymus, whose T-cell receptor (TCR) genes lack N regions and are encoded by Vgamma3-Jgamma1 and Vdelta1-Ddelta2-Jdelta2 with canonical junctions (invariant Vgamma3Vdelta1), are thought to be the precursors of dendritic epidermal T cells (DETC). We generated mutant mice whose endogenous TdT promoter was replaced with the lck promoter through homologous recombination. These mutant mice expressed TdT in fetal thymus, had abundant N regions and infrequent canonical junctions in gamma and delta rearrangements, and showed a decreased number of gammadelta T cells. Various Vgamma3Vdelta1 T cells, most of which had N regions in their TCR genes, were found to disseminate in the skin of newborn mutant mice, whereas normal numbers of DETCs with the invariant Vgamma3Vdelta1 rearrangement were observed in adult mutants. These data demonstrate that the regulation of TdT expression during fetal development is important for the generation of gammadelta T cells, and that Vgamma3Vdelta1 T cells, which have various junctional sequences in their TCR genes, randomly disseminate in skin, but invariant Vgamma3Vdelta1 T cells have a great advantage for proliferation in skin.
Figures


Similar articles
-
The role of short homology repeats and TdT in generation of the invariant gamma delta antigen receptor repertoire in the fetal thymus.Immunity. 1995 Oct;3(4):439-47. doi: 10.1016/1074-7613(95)90173-6. Immunity. 1995. PMID: 7584135
-
Positive selection of dendritic epidermal gammadelta T cell precursors in the fetal thymus determines expression of skin-homing receptors.Immunity. 2004 Jul;21(1):121-31. doi: 10.1016/j.immuni.2004.06.008. Immunity. 2004. PMID: 15345225
-
T cell receptor specificity is critical for the development of epidermal gammadelta T cells.J Exp Med. 2001 Nov 19;194(10):1473-83. doi: 10.1084/jem.194.10.1473. J Exp Med. 2001. PMID: 11714754 Free PMC article.
-
Generation of human gammadelta T-cell repertoires.Crit Rev Immunol. 1999;19(5-6):431-60. Crit Rev Immunol. 1999. PMID: 10647745 Review.
-
Functions of skin-resident γδ T cells.Cell Mol Life Sci. 2011 Jul;68(14):2399-408. doi: 10.1007/s00018-011-0702-x. Epub 2011 May 11. Cell Mol Life Sci. 2011. PMID: 21560071 Free PMC article. Review.
Cited by
-
Ontogenic timing, T cell receptor signal strength, and Notch signaling direct γδ T cell functional differentiation in vivo.Cell Rep. 2021 Jun 8;35(10):109227. doi: 10.1016/j.celrep.2021.109227. Cell Rep. 2021. PMID: 34107257 Free PMC article.
-
γδ T, NKT, and MAIT Cells During Evolution: Redundancy or Specialized Functions?J Immunol. 2022 Jul 15;209(2):217-225. doi: 10.4049/jimmunol.2200105. J Immunol. 2022. PMID: 35821101 Free PMC article. Review.
-
Inhibitor of DNA binding 3 limits development of murine slam-associated adaptor protein-dependent "innate" gammadelta T cells.PLoS One. 2010 Feb 19;5(2):e9303. doi: 10.1371/journal.pone.0009303. PLoS One. 2010. PMID: 20174563 Free PMC article.
-
The Role of Tissue-resident T Cells in Stress Surveillance and Tissue Maintenance.Cells. 2020 Mar 11;9(3):686. doi: 10.3390/cells9030686. Cells. 2020. PMID: 32168884 Free PMC article. Review.
-
Genetic architecture of innate and adaptive immune cells in pigs.Front Immunol. 2023 Feb 6;14:1058346. doi: 10.3389/fimmu.2023.1058346. eCollection 2023. Front Immunol. 2023. PMID: 36814923 Free PMC article.
References
-
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;14:575. - PubMed
-
- Lafaille J, Decloux A, Bonneville M, Takagaki Y, Tonegawa S. Junctional sequences of T cell receptor γδ gene: implications for γδ T cell lineages and for a novel intermediate of V-(D)-J joining. Cell. 1987;59:859. - PubMed
-
- Komori T, Okada A, Stewart V, Alt FW. Lack of N regions in antigen receptor variable region genes of TdT-deficient lymphocytes. Science. 1993;261:1171. - PubMed
-
- Gilfillan S, Dierich A, Lemeur M, Benoist C, Mathis D. Mice lacking TdT: mature animals with an immature lymphocyte repertoire. Science. 1993;261:1175. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

