Antibody cross-linking of human CD9 and the high-affinity immunoglobulin E receptor stimulates secretion from transfected rat basophilic leukaemia cells
- PMID: 10792502
- PMCID: PMC2327194
- DOI: 10.1046/j.1365-2567.2000.00992.x
Antibody cross-linking of human CD9 and the high-affinity immunoglobulin E receptor stimulates secretion from transfected rat basophilic leukaemia cells
Abstract
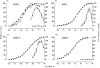
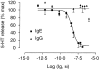
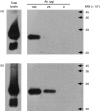
Previous studies have shown that antibody cross-linking of the tetraspanin protein CD9 stimulates the degranulation of platelets and eosinophils, although the mechanism of activation is unclear. In this work we transfected human CD9 into the rat basophilic leukaemia (RBL-2H3) cell line and studied the stimulation of secretion from these cells in response to a panel of anti-CD9 antibodies. Intact immunoglobulin G1 (IgG1) antibodies activated transfected cells whereas F(ab')2 fragments of antibody and an intact IgG2a did not. Stimulation of secretion was inhibited by co-incubation with monomer murine immunoglobulin E (IgE) but not with an IgG1 isotype control, indicating that the response involves the endogenous high-affinity IgE receptor (FcepsilonRI). The anti-CD9 antibody activation curve was biphasic, and supraoptimal antibody concentrations stimulated little or no degranulation, indicating that multivalent binding of human CD9 molecules is necessary for the formation of an active complex with rat FcepsilonRI. Immunoprecipitation of FcepsilonRI under mild detergent conditions co-precipitated CD9, suggesting the presence of pre-existing complexes of CD9 and FcepsilonRI that could be activated by antibody cross-linking. These data are further evidence that tetraspanins are involved in FcepsilonRI signalling and may reflect the participation of tetraspanins in the formation of complexes with other membrane proteins that use components of Fc receptors for signal transduction.
Figures
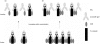
Similar articles
-
Cross-linking of Thy-1 glycoproteins or high-affinity IgE receptors induces mast cell activation via different mechanisms.Immunology. 1993 Sep;80(1):103-9. Immunology. 1993. PMID: 7902332 Free PMC article.
-
An immunoglobulin E-reactive chimeric human immunoglobulin G1 anti-idiotype inhibits basophil degranulation through cross-linking of FcepsilonRI with FcgammaRIIb.Clin Exp Allergy. 2008 Feb;38(2):313-9. doi: 10.1111/j.1365-2222.2007.02896.x. Epub 2007 Dec 7. Clin Exp Allergy. 2008. PMID: 18070161
-
Antibodies against human CD63 activate transfected rat basophilic leukemia (RBL-2H3) cells.Mol Immunol. 1995 Dec;32(17-18):1339-44. doi: 10.1016/0161-5890(95)00113-1. Mol Immunol. 1995. PMID: 8643103
-
The effectiveness of different rat IgG subclasses as IgE-blocking antibodies in the rat basophil leukaemia cell model.Immunol Cell Biol. 1999 Apr;77(2):121-6. doi: 10.1046/j.1440-1711.1999.00801.x. Immunol Cell Biol. 1999. PMID: 10234546
-
Review: Diagnostic and therapeutic applications of rat basophilic leukemia cells.Mol Immunol. 2012 Oct;52(3-4):224-8. doi: 10.1016/j.molimm.2012.05.019. Epub 2012 Jun 27. Mol Immunol. 2012. PMID: 22750069 Review.
Cited by
-
CD9 Tetraspanin: A New Pathway for the Regulation of Inflammation?Front Immunol. 2018 Oct 9;9:2316. doi: 10.3389/fimmu.2018.02316. eCollection 2018. Front Immunol. 2018. PMID: 30356731 Free PMC article. Review.
-
CD9+ Regulatory B Cells Induce T Cell Apoptosis via IL-10 and Are Reduced in Severe Asthmatic Patients.Front Immunol. 2018 Dec 21;9:3034. doi: 10.3389/fimmu.2018.03034. eCollection 2018. Front Immunol. 2018. PMID: 30622536 Free PMC article.
-
Tetraspanins and Transmembrane Adaptor Proteins As Plasma Membrane Organizers-Mast Cell Case.Front Cell Dev Biol. 2016 May 10;4:43. doi: 10.3389/fcell.2016.00043. eCollection 2016. Front Cell Dev Biol. 2016. PMID: 27243007 Free PMC article. Review.
-
A role for tetraspanin proteins in regulating fusion induced by Burkholderia thailandensis.Med Microbiol Immunol. 2020 Aug;209(4):473-487. doi: 10.1007/s00430-020-00670-6. Epub 2020 Apr 6. Med Microbiol Immunol. 2020. PMID: 32253503 Free PMC article.
-
Tetraspanins in mast cells.Front Immunol. 2012 May 7;3:106. doi: 10.3389/fimmu.2012.00106. eCollection 2012. Front Immunol. 2012. PMID: 22783251 Free PMC article.
References
-
- Wright MD, Tomlinson MG. The ins and outs of the transmembrane-4 superfamily. Immunol Today. 1994;15:588. - PubMed
-
- RubeIstein E, Lenaour F, Lagaudriere-gesbert C, Billared M, Conjeaud H, Boucheix C. CD9, CD63, CD81, and CD82 are components of a surface tetraspan network connected to HLA-DR and VLA integrins. Eur J Immunol. 1996;26:2657. - PubMed
-
- Hemler ME, Mannion BA, Berditchevski F. Association of TM4SF proteins with integrins – relevance to cancer. Biochim Biophys Acta. 1996;1287:67. - PubMed
-
- Maecker HT, Todd SC, Levy S. The tetraspanin superfamily: molecular facilitators. FASEB J. 1997;11:428. - PubMed
-
- Jones PH, Bishop LA, Watt FM. Functional significance of CD9 association with beta (1) integrins in human epidermal keratinocytes. Cell Adhesion Commun. 1996;4:297. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

