Complement activation after oxidative stress: role of the lectin complement pathway
- PMID: 10793066
- PMCID: PMC1876913
- DOI: 10.1016/S0002-9440(10)65026-2
Complement activation after oxidative stress: role of the lectin complement pathway
Abstract
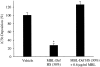
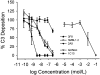
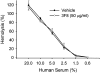
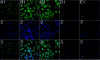
The complement system plays an important role in mediating tissue injury after oxidative stress. The role of mannose-binding lectin (MBL) and the lectin complement pathway (LCP) in mediating complement activation after endothelial oxidative stress was investigated. iC3b deposition on hypoxic (24 hours; 1% O(2))/reoxygenated (3 hours; 21% O(2)) human endothelial cells was attenuated by N-acetyl-D-glucosamine or D-mannose, but not L-mannose, in a dose-dependent manner. Endothelial iC3b deposition after oxidative stress was also attenuated in MBL-deficient serum. Novel, functionally inhibitory, anti-human MBL monoclonal antibodies attenuated MBL-dependent C3 deposition on mannan-coated plates in a dose-dependent manner. Treatment of human serum with anti-MBL monoclonal antibodies inhibited MBL and C3 deposition after endothelial oxidative stress. Consistent with our in vitro findings, C3 and MBL immunostaining throughout the ischemic area at risk increased during rat myocardial reperfusion in vivo. These data suggest that the LCP mediates complement activation after tissue oxidative stress. Inhibition of MBL may represent a novel therapeutic strategy for ischemia/reperfusion injury and other complement-mediated disease states.
Figures




References
-
- Weisman HF, Bartow T, Leppo MK, Marsh HC, Jr, Carson GR, Concino MF, Boyle MP, Roux KH, Weisfeldt ML, Fearon DT: Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science 1990, 249:146-151 - PubMed
-
- Collard CD, Vakeva A, Bukusoglu C, Zünd G, Sperati CJ, Colgan SP, Stahl GL: Reoxygenation of hypoxic human umbilical vein endothelial cells activates the classic complement pathway. Circulation 1997, 96:326-333 - PubMed
-
- Collard CD, Agah A, Stahl GL: Complement activation following reoxygenation of hypoxic human endothelial cells: role of intracellular reactive oxygen species, NF-κB and new protein synthesis. Immunopharmacology 1998, 39:39-50 - PubMed
-
- Collard CD, Agah A, Reenstra WR, Buras JA, Stahl GL: Endothelial nuclear factor-κB translocation and vascular cell adhesion molecule-1 induction by complement: Inhibition with anti-C5 therapy or cGMP analogues. Arterioscler Thromb Vasc Biol 1999, 19:2623-2629 - PubMed
-
- Buerke M, Murohara T, Lefer AM: Cardioprotective effects of a C1 esterase inhibitor in myocardial ischemia and reperfusion. Circulation 1995, 91:393-402 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

