Differential nuclear and cytoplasmic expression of PTEN in normal thyroid tissue, and benign and malignant epithelial thyroid tumors
- PMID: 10793080
- PMCID: PMC1876937
- DOI: 10.1016/S0002-9440(10)65040-7
Differential nuclear and cytoplasmic expression of PTEN in normal thyroid tissue, and benign and malignant epithelial thyroid tumors
Abstract
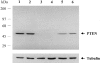
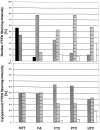
Germline mutations in PTEN (MMAC1/TEP1) are found in patients with Cowden syndrome, a familial cancer syndrome which is characterized by a high risk of breast and thyroid neoplasia. Although somatic intragenic PTEN mutations have rarely been found in benign and malignant sporadic thyroid tumors, loss of heterozygosity (LOH) has been reported in up to one fourth of follicular thyroid adenomas (FAs) and carcinomas. In this study, we examined PTEN expression in 139 sporadic nonmedullary thyroid tumors (55 FA, 27 follicular thyroid carcinomas, 35 papillary thyroid carcinomas, and 22 undifferentiated thyroid carcinomas) using immunohistochemistry and correlated this to the results of LOH studies. Normal follicular thyroid cells showed a strong to moderate nuclear or nuclear membrane signal although the cytoplasmic staining was less strong. In FAs the neoplastic nuclei had less intense PTEN staining, although the cytoplasmic PTEN-staining intensity did not differ significantly from that observed in normal follicular cells. In thyroid carcinomas as a group, nuclear PTEN immunostaining was mostly weak in comparison with normal thyroid follicular cells and FAs. The cytoplasmic staining was more intense than the nuclear staining in 35 to 49% of carcinomas, depending on the histological type. Among 81 informative tumors assessed for LOH, there seemed to be an associative trend between decreased nuclear and cytoplasmic staining and 10q23 LOH (P = 0.003, P = 0.008, respectively). These data support a role for PTEN in the pathogenesis of follicular thyroid tumors.
Figures

References
-
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R: PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 1997, 1943, 275:277 - PubMed
-
- Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng DH, Tavtigian SV: Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet 1997, 15:356-362 - PubMed
-
- Li DM, Sun H: TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res 1997, 57:2124-2129 - PubMed
-
- Teng DH, Hu R, Lin H, Davis T, Iliev D, Frye C, Swedlund B, Hansen KL, Vinson VL, Gumpper KL, Ellis L, El-Naggar A, Frazier M, Jasser S, Langford LA, Lee J, Mills GB, Pershouse MA, Pollack RE, Tornos C, Troncoso P, Yung WK, Fujii G, Berson A, Bookstein R, Bolen JB, Tavtigian SV, Steck PA: MMAC1/PTEN mutations in primary tumor specimens and tumor cell lines. Cancer Res 1997, 57:5221-5225 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

