Identification of a novel member of the chloride intracellular channel gene family (CLIC5) that associates with the actin cytoskeleton of placental microvilli
- PMID: 10793131
- PMCID: PMC14863
- DOI: 10.1091/mbc.11.5.1509
Identification of a novel member of the chloride intracellular channel gene family (CLIC5) that associates with the actin cytoskeleton of placental microvilli
Abstract
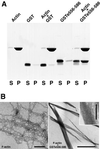
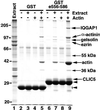
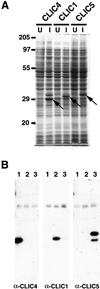
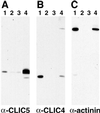
The chloride intracellular channel (CLIC) gene family has been implicated in chloride ion transport within various subcellular compartments. We report here the molecular, biochemical, and cellular characterization of a new member of this gene family termed CLIC5. CLIC5 was isolated from extracts of placental microvilli as a component of a multimeric complex consisting of several known cytoskeletal proteins, including actin, ezrin, alpha-actinin, gelsolin, and IQGAP1. We cloned human cDNAs and generated antibodies specific for CLIC5, CLIC1/NCC27, and CLIC4/huH1/p64H1. CLIC5 shares 52-76% overall identity with human CLIC1, CLIC2, CLIC3, and CLIC4. Northern blot analysis showed that CLIC5 has a distinct pattern of expression compared with CLIC1 and CLIC4. Immunoblot analysis of extracts from placental tissues demonstrated that CLIC4 and CLIC5 are enriched in isolated placental microvilli, whereas CLIC1 is not. Moreover, in contrast to CLIC1 and CLIC4, CLIC5 is associated with the detergent-insoluble cytoskeletal fraction of microvilli. Indirect immunofluorescence microscopy revealed that CLIC4 and CLIC5 are concentrated within the apical region of the trophoblast, whereas CLIC1 is distributed throughout the cytoplasm. These studies suggest that CLIC1, CLIC4, and CLIC5 play distinct roles in chloride transport and that CLIC5 interacts with the cortical actin cytoskeleton in polarized epithelial cells.
Figures





References
-
- Al-Awqati Q. Chloride channels of intracellular organelles. Curr Opin Cell Biol. 1995;7:504–508. - PubMed
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Begenisich T, Melvin JE. Regulation of chloride channels in secretory epithelia. J Membr Biol. 1998;163:77–85. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

