High-resolution FRET microscopy of cholera toxin B-subunit and GPI-anchored proteins in cell plasma membranes
- PMID: 10793141
- PMCID: PMC14873
- DOI: 10.1091/mbc.11.5.1645
High-resolution FRET microscopy of cholera toxin B-subunit and GPI-anchored proteins in cell plasma membranes
Abstract
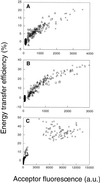
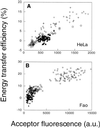
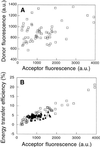
"Lipid rafts" enriched in glycosphingolipids (GSL), GPI-anchored proteins, and cholesterol have been proposed as functional microdomains in cell membranes. However, evidence supporting their existence has been indirect and controversial. In the past year, two studies used fluorescence resonance energy transfer (FRET) microscopy to probe for the presence of lipid rafts; rafts here would be defined as membrane domains containing clustered GPI-anchored proteins at the cell surface. The results of these studies, each based on a single protein, gave conflicting views of rafts. To address the source of this discrepancy, we have now used FRET to study three different GPI-anchored proteins and a GSL endogenous to several different cell types. FRET was detected between molecules of the GSL GM1 labeled with cholera toxin B-subunit and between antibody-labeled GPI-anchored proteins, showing these raft markers are in submicrometer proximity in the plasma membrane. However, in most cases FRET correlated with the surface density of the lipid raft marker, a result inconsistent with significant clustering in microdomains. We conclude that in the plasma membrane, lipid rafts either exist only as transiently stabilized structures or, if stable, comprise at most a minor fraction of the cell surface.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

