A novel selection regime for differentiation defects demonstrates an essential role for the stumpy form in the life cycle of the African trypanosome
- PMID: 10793160
- PMCID: PMC14892
- DOI: 10.1091/mbc.11.5.1905
A novel selection regime for differentiation defects demonstrates an essential role for the stumpy form in the life cycle of the African trypanosome
Abstract
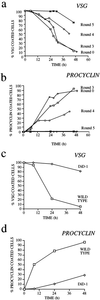
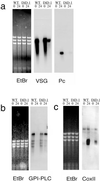
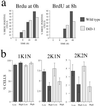
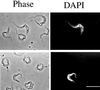
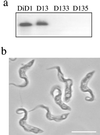
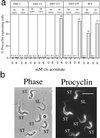
A novel selection scheme has been developed to isolate bloodstream forms of Trypanosoma brucei, which are defective in their ability to differentiate to the procyclic stage. Detailed characterization of one selected cell line (defective in differentiation clone 1 [DiD-1]) has demonstrated that these cells are indistinguishable from the wild-type population in terms of their morphology, cell cycle progression, and biochemical characteristics but are defective in their ability to initiate differentiation to the procyclic form. Although a small proportion of DiD-1 cells remain able to transform, deletion of the genes for glycophosphatidyl inositol-phospholipase C demonstrated that this enzyme was not responsible for this inefficient differentiation. However, the attenuated growth of the Delta-glycophosphatidyl inositol-phospholipase C DiD-1 cells in mice permitted the expression of stumpy characteristics in this previously monomorphic cell line, and concomitantly their ability to differentiate efficiently was restored. Our results indicate that monomorphic cells retain expression of a characteristic of the stumpy form essential for differentiation, and that this is reduced in the defective cells. This approach provides a new route to dissection of the cytological and molecular basis of life cycle progression in the African trypanosome.
Figures


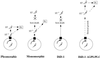
References
-
- Ashcroft MT. A comparison between a syringe passaged and a tsetse fly transmitted line of a strain of Trypanosoma rhodesiense. Ann Trop Med Parasitol. 1960;54:44–70. - PubMed
-
- Bass KE, Wang CC. The in vitro differentiation of pleomorphic Trypanosoma brucei from bloodstream into procyclic form requires neither intermediary nor short-stumpy stage. Mol Biochem Parasitol. 1991;44:261–270. - PubMed
-
- Bass KE, Wang CC. Transient inhibition of protein synthesis accompanies differentiation of Trypanosoma brucei from bloodstream to procyclic forms. Mol Biochem Parasitol. 1992;56:129–140. - PubMed
-
- Brown RC, Evans DA, Vickerman K. Changes in oxidative metabolism and ultrastructure accompanying differentiation of the mitochondrion in Trypanosoma brucei. Int JParasitol. 1973;3:691–704. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

