Betamimetics for suspected impaired fetal growth
- PMID: 10796095
- PMCID: PMC7052749
- DOI: 10.1002/14651858.CD000036
Betamimetics for suspected impaired fetal growth
Update in
-
Betamimetics for suspected impaired fetal growth.Cochrane Database Syst Rev. 2001;(4):CD000036. doi: 10.1002/14651858.CD000036. Cochrane Database Syst Rev. 2001. PMID: 11687064
Abstract
Background: Betamimetic drugs may promote fetal growth by increasing the availability of nutrients and by decreasing vascular resistance. They may also induce adverse effects via their effects on carbohydrate metabolism.
Objectives: The objective of this review was to assess the effects of betamimetic therapy for suspected impaired fetal growth on fetal growth and perinatal outcome.
Search strategy: We searched the Cochrane Pregnancy and Childbirth Group trials register and the Cochrane Controlled Trials Register. Date of the latest search: December 1999.
Selection criteria: Randomised trials of betamimetic therapy compared with no betamimetic therapy or placebo in women with suspected impaired fetal growth.
Data collection and analysis: Eligibility and trial quality was assessed.
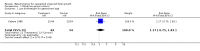
Main results: Two studies of 118 women were included. No differences were found between the betamimetic groups and the control groups for low birth weight (relative risk 1.17, 95% confidence interval 0.75 to 1.83), other anthropometric or neonatal morbidity and mortality measures.
Reviewer's conclusions: There is not enough evidence to evaluate the use of betamimetics in promoting fetal growth. Larger studies are needed to investigate possible adverse effects of long-term betamimetic administration.
Conflict of interest statement
None known.
Figures





References
References to studies included in this review
Cabero 1988 {published data only}
-
- Cabero L, Cerqueira MJ, Solar J, Bellart J, Esteban‐Altirriba J. Long‐term hospitalization and beta‐mimetic therapy in the treatment of intrauterine growth retardation of unknown etiology. Journal of Perinatal Medicine 1988;16:453‐8. - PubMed
Mori 1990 {published data only}
-
- Mori H, Aisaka K, Arai K, Okinaga S. Trial of ritodrine infusion for treatment of intrauterine growth retardation. Proceedings of 6th Congress of the Federation of the Asia‐Oceania Perinatal Societies; 1990 Oct 22‐26; Perth, Western Australia. 1990:46.
References to studies excluded from this review
Chang 1977 {published data only}
-
- Chang A, Abell D, Beischer N, Wood C. Trial of intravenous therapy in women with low urinary oestriol excretion. American Journal of Obstetrics and Gynecology 1977;127:793‐7. - PubMed
Grant 1987 {unpublished data only}
-
- Grant AM. Oral salbutamol in the prevention of low birthweight in singleton pregnancies. Personal communication January 17 1987.
Renaud 1972 {published data only}
-
- Brettes JP, Renaud R, Gandar R. A double‐blind investigation into the effects of ritodrine on uterine blood flow during the third trimester of pregnancy. American Journal of Obstetrics and Gynecology 1976;124:164‐8. - PubMed
-
- Renaud R, Brettes P, Irrmann M, Boog G, Schumacher J, Gandar R. A double‐blind cross‐over trial to assess the effect of ritodrine on uterine blood flow in normal and pathologic pregnancies. A preliminary report. Proceedings of International Symposium on the Treatment of Fetal Risks; 1972 May; Baden, Austria. 1972:53‐4.
Renaud 1976 {published data only}
-
- Renaud R, Bock A, Chambron J, Bonafous J, Rosenthal C, Raffi F. A simultaneous study of the placental, myometrial and cervical circulations (using Indium 113 and anemometric thermometry). A double blind study of the action of intravenous ritodrine in normal pregnancy. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 1976;5:611‐5. - PubMed
Spellacy 1978 {published data only}
-
- Spellacy WN, Cruz AC, Buhi WC, Birk SA. The acute effects of ritodrine infusion on maternal metabolism: measurement of levels of glucose, insulin, glucagon, triglycerides, cholesterol, placental lactogen and chorionic gonadotropin. American Journal of Obstetrics and Gynecology 1978;131:637‐42. - PubMed
Varma 1987 {unpublished data only}
-
- Varma TR. A randomised double‐blind comparison of ritodrine vs placebo in the treatment of intrauterine growth retardation. Personal communication September 29 1987.
Additional references
Bernstein 2000
-
- Bernstein IM, Horbar, JD, Badger GJ, Ohlsson A, Golan A. Morbidity and mortality among very low birth weight infants with intrauterine growth restriction. The Vermont Oxford Network. American Journal of Obstetrics and Gynecology 2000;182:198. - PubMed
Clarke 2000
-
- Clarke M, Oxman AD, editors. Cochrane Reviewers' Handbook 4.1 [updated June 2000]. In: The Cochrane Library [database on CDROM]. The Cochrane Collaboration. Oxford: Update Software; 2000, Issue 2.
Harding 1992
-
- Harding JE, Owens JA, Robinson JS. Should we try to supplement the growth retarded fetus? A cautionary tale. British Journal of Obstetrics and Gynaecology 1992;99:707‐10. - PubMed
Lin 1998
-
- Lin C, Santolaya‐Forgas J. Current concepts of fetal growth restriction: Part 1. Causes, classification, and pathophysiology. Obstetrics & Gynecology 1998;92(6):1044‐55. - PubMed
Resnik 2002
-
- Resnik R. Intrauterine growth restriction. Obstetrics & Gynecology 2002;99(3):490‐6. - PubMed
RevMan 2000 [Computer program]
-
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.1 for Windows. Oxford, England: The Cochrane Collaboration, 2000.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

