Furosemide for symptomatic patent ductus arteriosus in indomethacin-treated infants
- PMID: 10796253
- PMCID: PMC7032649
- DOI: 10.1002/14651858.CD001148
Furosemide for symptomatic patent ductus arteriosus in indomethacin-treated infants
Update in
-
Furosemide for symptomatic patent ductus arteriosus in indomethacin-treated infants.Cochrane Database Syst Rev. 2001;(3):CD001148. doi: 10.1002/14651858.CD001148. Cochrane Database Syst Rev. 2001. PMID: 11686979
Abstract
Background: Inhibition of prostaglandin synthesis mediates closure of the ductus arteriosus and renal side effects after indomethacin administration. Because furosemide increases prostaglandin production, it could potentially help prevent indomethacin-related toxicity but also decrease ductal response to indomethacin.
Objectives: The primary objectives of this review were to assess (1) whether furosemide affects the incidence of failure of ductal closure after indomethacin and that of indomethacin-related toxicity and (2) the effect of furosemide on mid-term and long-term outcome. The secondary objective was to determine whether the effect of furosemide on renal function and water balance depends on prior extracellular volume (assessed by blood urea nitrogen [BUN]/creatinine ratio).
Search strategy: We searched electronic databases (Medline, Embase and Cochrane) and selected abstract books, without language restriction.
Selection criteria: We selected studies with (1) random allocation to either indomethacin alone or indomethacin and furosemide and (2) analysis of either short-term risk-benefit ratio of furosemide, mid- or long-term outcome, or the relationship between extracellular volume at study entry and changes in renal function.
Data collection and analysis: We assessed studies for possible bias and for quality of assessment of ductal patency. We assessed categorical variables using relative risk and absolute risk reduction. We assessed the effects of furosemide on renal function and fluid balance by comparing changes from baseline in the treatment group with those in controls. Subsets were determined a priori based on BUN/creatinine ratio at study entry.
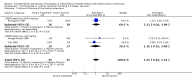
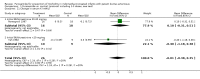
Main results: All 3 studies fulfilling the entry criteria had limitations, including possible or definite bias. There was substantial heterogeneity among studies. Furosemide administration did not significantly increase the risk of failure of ductal closure; however, sample size was insufficient to rule out even a 31% increase. In the subset with initial BUN/creatinine ratio > 20 mg/mg, 2 of 18 patients receiving furosemide could not complete a 3-dose course of indomethacin because of toxicity. Minimal or no information was available about any of the other main outcome variables. Furosemide increased urine output regardless of the initial BUN/creatinine ratio, leading to a 5% weight loss during a 3-dose course, an undesired effect in patients with initial BUN/creatinine ratio > 20 mg/mg. Furosemide increased creatinine clearance only in patients with initial BUN/creatinine ratio <20 mg/mg.
Reviewer's conclusions: There is not enough evidence to support the administration of furosemide to premature infants treated with indomethacin for symptomatic patent ductus arteriosus. Furosemide appears to be contraindicated in the presence of dehydration in those infants.
Conflict of interest statement
None
Figures























References
References to studies included in this review
Romagnoli 1997 {published data only}
-
- Romagnoli C, Zecca E, Papacci P, Carolis MP, Giannini R, Gallini F, et. al. Furosemide does not prevent indomethacin‐induced renal side effects in preterm infants. Clinical Pharmacology and Therapeutics 1997;62:181‐6. [MEDLINE: ] - PubMed
Vargas‐Origel 1986 {published data only}
-
- Vargas‐Origel A, Cruz‐Anguiano V, López‐Montaño A. Indometacina y furosemide en el cierre del conducto arterioso. Boletin Medico del Hospital Infantil de Mexico 1986;43:482‐8. [MEDLINE: ] - PubMed
Yeh 1982 {published data only}
-
- Yeh TF, Wilks A, Singh J, Betkerur M, Lilien L, Pildes RS. Furosemide prevents the renal side effects of indomethacin therapy in premature infants with patent ductus arteriosus. Journal of Pediatrics 1982;101:433‐7. [MEDLINE: ] - PubMed
References to studies excluded from this review
Barrington 1994 {published data only}
Maslarska 1997 {published data only}
-
- Maslarska R, Spasov L, Klyasova R, Tomova S. Therapeutic conduct in preterm neonates with patent ductus arteriosus persistens and respiratory distress syndrome (RDS). Pediatriya 1997;36:22‐3. [MEDLINE: ]
Merritt 1981 {published data only}
-
- Merritt TA, Harris JP, Roghmann K, Wood B, Campanella V, Alexson C, et. al. Early closure of the patent ductus arteriosus in very‐low‐birth‐weight infants: A controlled trial. Journal of Pediatrics 1981;99:281‐6. [MEDLINE: ] - PubMed
Neubauer 1995 {published data only}
-
- Neubauer AP, Müller‐Deile K. Verschluss des hämodynamisch wirksamen persistierenden Ductus Botalli (PDA) beim Frühgeborenen unter 1000 G mit Indometacin. Monatsschrift fur Kinderheilkunde 1995;143:1224‐30. [MEDLINE: ]
Petion 1990 {published data only}
-
- Pétion AM, Gouyon JP, Sandré D. Le furosémide prévient‐il les perturbations des fonctions rénales induites par l'administration d'indométacine chez le prématuré?. Archives Francaises de Pédiatrie 1990;47:615‐6. [MEDLINE: ] - PubMed
Ransom 1990 {published data only}
-
- Ransom JL, Gal P, Weaver RL, Schall S, Brown Y. Impact of concurrent furosemide on indomethacin pharmacokinetics Re: Efficacy for patent ductus arteriosus closure and renal toxicity. American Journal of Perinatology 1990;7:393.
Yeh 1986 {published data only}
-
- Yeh TF, Wilks A, Luken J, Pildes RS. Indomethacin therapy in premature infants with patent ductus arteriosus and oliguria. Developmental Pharmacology and Therapeutics 1986;9:369‐74. [MEDLINE: ] - PubMed
-
- Yeh TF, Wilks A, Raval D, Pildes RS. Furosemide prevents the renal side effects of indomethacin in premature infants with PDA and oliguria. Pediatric Research 1985;19:181A. - PubMed
Additional references
Alpert 1979
-
- Alpert BS, Lewins MJ, Rowland DW, Grant MJ, Olle PM, Soldin SJ, et. al. Plasma indomethacin levels in preterm newborn infants with symptomatic patent ductus arteriosus ‐ clinical and echocardiographic assessments of response. Journal of Pediatrics 1979;95:578‐82. - PubMed
Armitage 1994
-
- Armitage P, Berry G. Sampling. In: Armitage P, Berry G editor(s). Statistical Methods in Medical Research. Third Edition. Oxford: Blackwell Scientific Publications, 1994:78‐92.
Attallah 1979
-
- Attallah A, Stahl R, Bloch D, Lee JB. Furosemide stimulates and saralasin inhibits renal prostaglandin E2 biosynthesis. Clinical Research 1979;27:559A.
Baird 1995
-
- Baird DC. Statistics of observation. In: Baird DC editor(s). Experimentation. An introduction to measurement theory and experimental design. New Jersey: Prentice Hall, 1994:29‐56.
Bergamo 1989
-
- Bergamo RR, Cominellli F, Kopple JD, Zipser RD. Comparative acute effects of aspirin, diflunisal, ibuprofen and indomethacin on renal function in healthy man. American Journal of Nephrology 1989;9:460‐3. [MEDLINE: ] - PubMed
Brion 1994
-
- Brion LP, Satlin LM, Edelmann CM Jr. Renal disease. In: Avery GB, Fletcher MA, MacDonald MG editor(s). Neonatology: Pathophysiology and management of the newborn. Philadelphia: JB Lippincott Co, 1994:792‐886.
Christmann 1998
-
- Christmann V, Semmekrot BA, Bor M. Changes in cerebral, renal and mesenteric blood flow velocity during continuous and bolus infusion of indomethacin [abstract]. Pediatric Research 1998;43:169A. - PubMed
Cochran 1989
-
- Cochran J, Reddy R, Devaskar U. Effect of indomethacin (I) vs I + dopamine on serum BUN and creatinine, urinary output and the closure of patent ductus arteriosus in preterm neonates with hyaline membrane disease. Pediatric Research 1989;25:211A.
Daskalopoulos 1985
-
- Daskalopoulos G, Kronborg I, Katkov W, Gonzalez M, Laffi G, Zipser RD. Sulindac and indomethacin suppress the diuretic action of furosemide in patients with cirrhosis and ascites: evidence that sulindac affects renal prostaglandins. American Journal of Kidney Diseases 1985;6:217‐21. [MEDLINE: ] - PubMed
Evans 1981
-
- Evans M, Bhat R, Vidyasagar D, Patel M, Hastreiter A. A comparison of oral and intravenous indomethacin dispositions in the premature infant with patent ductus arteriosus. Pediatric Pharmacology 1981;1:251‐8. - PubMed
Follmann 1992
-
- Zimmerman JJ. Bronchoalveolar inflammatory pathophysiology of bronchopulmonary dysplasia. Clinics in Perinatology 1995;22:429‐56. - PubMed
Fowlie 1997
-
- Fowlie PW. Prophylactic intravenous indomethacin in very low birth infants. Cochrane Database of Systematic Reviews 1998, Issue 3. [DOI: 10.1002/14651858.CD000174] - DOI
Friedman 1982
-
- Friedman CA, Parks BR, Rawson JE, Serwer GA, Anderson PAW. Indomethacin and the preterm infant with a patent ductus arteriosus: Relationship between plasma concentration and ductus closure. Developmental Pharmacology and Therapeutics 1982;4:37‐46. [MEDLINE: ] - PubMed
Green 1983
-
- Green TP, Thompson TR, Johnson De, Lock JE. Furosemide promotes patent ductus arteriosus in premature infants with the respiratory‐distress syndrome. New England Journal of Medicine 1983;308:743‐8. [MEDLINE: ] - PubMed
Kahles 1987
-
- Kahles H, Riegger AJ. Indometacin und Furosemid bei Patienten mit Herzinsuffizienz. Nierenfunktion, Renin‐Angiotensin‐System und renale Prostaglandine. Deutsche Medizinische Wochenschrift 1987;112:1737‐40. [MEDLINE: ] - PubMed
Leititis 1987
-
- Leititis JU, Burghard R, Gordjani N, Wildberg A, Seyberth HW, Brandis M. Effect of a modified fluid therapy on renal function during indomethacin therapy for persistent ductus arteriosus. Acta Paediatrica Scandinavica 1987;76:789‐94. [MEDLINE: ] - PubMed
Lin 1978
-
- Lin YM, Jarabak J. Isolation of two proteins with 9‐ketoprostaglandin reductase and NADP‐linked 15‐hydroxyprostaglandin dehydrogenase activities and studies on their inhibition. Biochemical and Biophysical Research Communications 1978;81:1227‐34. [MEDLINE: ] - PubMed
Lin 1995
-
- Lin YJ, Tsai YJ, Chen JS, Lin JS, Wu JM, Lin CH, et. al. Renal effects and urinary excretion of prostaglandin following indomethacin therapy in premature infants with patent ductus arteriosus. Acta Paediatrica Sinica 1995;36:104‐7. [MEDLINE: ] - PubMed
Mirouze 1983
-
- Mirouze D, Zipser RD, Reynolds TB. Effect of inhibitors of prostaglandin synthesis on induced diuresis in cirrhosis. Hepatology 1983;3:50‐5. [MEDLINE: ] - PubMed
Mrongovius 1982
-
- Mrongovius R, Imbeck H, Wille L, Muller H, Seyberth HW. Variability of serum indomethacin concentrations after oral and intravenous administration to preterm infants. European Journal of Pediatrics 1982;138:151‐3. - PubMed
Seri 1984
-
- Seri I, Tulassay T, Kiszel J, Csomor S. The use of dopamine for the prevention of the renal side effects of indomethacin in premature infants with patent ductus arteriosus. International Journal of Pediatric Nephrology 1984;5:209‐14. [MEDLINE: ] - PubMed
Seyberth 1983
-
- Seyberth HW, Rascher W, Hackenthal R, Wille L. Effect of prolonged indomethacin therapy on renal function and selected vasoactive hormones in very‐low‐birth‐weight infants with symptomatic patent ductus arteriosus. Journal of Pediatrics 1983;103:979‐84. [MEDLINE: ] - PubMed
Stone 1976
-
- Stone KJ, Hart M. Inhibition of renal PGE2‐9‐ketoreductase by diuretics. Prostaglandins 1976;12:197‐207. [MEDLINE: ] - PubMed
van Bel 1991
-
- Bel F, Guit GL, Schipper J, Bor M, Baan J. Indomethacin‐induced changes in renal blood flow velocity waveform in premature infants investigated with color Doppler imaging. Journal of Pediatrics 1991;118:621‐6. [MEDLINE: ] - PubMed
Zecca 1994
-
- Zecca E, Papacci P, Carolis MP, Pasquini R, Vento G, Romagnoli C. Il trattamento dell pervieta del dotto arterioso con indometacina nel neonato pretermine: confronto tra due diverse modalita di infusione. Riv Ital Pediatr (IJP) 1994;20:228‐33.
References to other published versions of this review
Brion 1998a
-
- Brion LP, Campbell DE. Furosemide in indomethacin‐treated infants with symptomatic ductus arteriosus. Cochrane Database of Systematic Reviews 1998, Issue 3. [DOI: 10.1002/14651858.CD001148] - DOI
Brion 1998b
-
- Brion LP, Campbell DE. Furosemide in indomethacin‐treated premature infants with symptomatic patent ductus arteriosus ‐ Systematic review and meta‐analysis. Journal of the American Society of Nephrology. 1998; Vol. 9:141A.
Brion 1999
-
- Brion LP, Campbell DE. Furosemide in indomethacin‐treated infants. Systematic review and meta‐analysis. Pediatric Nephrology 1999;13:212‐8. - PubMed
Brion 2001
-
- Brion LP, Campbell DE. Furosemide for prevention of morbidity in indomethacin‐treated infants with patent ductus arteriosus. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD001148] - DOI
Brion 2004
-
- Brion LP, Campbell DE. Furosemide for prevention of morbidity in indomethacin‐treated infants with patent ductus arteriosus. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD001148] - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

