Fully intermittent dosing with drugs for tuberculosis
- PMID: 10796561
- PMCID: PMC6532565
- DOI: 10.1002/14651858.CD000970
Fully intermittent dosing with drugs for tuberculosis
Update in
-
Fully intermittent dosing with drugs for tuberculosis.Cochrane Database Syst Rev. 2000;(4):CD000970. doi: 10.1002/14651858.CD000970. Cochrane Database Syst Rev. 2000. Update in: Cochrane Database Syst Rev. 2001;(4):CD000970. doi: 10.1002/14651858.CD000970. PMID: 11034692 Updated.
Abstract
Background: The number of people infected with tuberculosis continues to rise world-wide. Rifampicin-containing treatment regimens can achieve high cure rates. Intermittent drug treatment delivered in the community has the potential to improve adherence to treatment.
Objectives: The objective of this review was to compare the effectiveness of rifampicin-containing short-course chemotherapy regimens, given two or three times a week, with similar regimens given daily in patients with pulmonary tuberculosis.
Search strategy: We searched the Cochrane Infectious Diseases Group trials register, the Cochrane Controlled Trials Register, Medline, and reference lists of articles. We contacted experts in the field.
Selection criteria: Randomised and quasi-randomised trials of any multi-drug regimen containing rifampicin in patients with confirmed pulmonary tuberculosis. Treatment had to be given up to three times a week for up to nine months, with any initial daily dosing period not more than one month, and was compared to daily dosing throughout for the same period.
Data collection and analysis: Two reviewers independently assessed trial eligibility and quality.
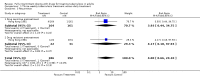
Main results: One trial involving 399 patients was included. The trial compared treatment three times per week with daily treatment for six months. There was no difference in cure rate (198 out of 199 people in the intermittent group compared to all 200 in the daily group), but 5 patients relapsed in the group receiving intermittent therapy compared to one in the group receiving the daily regimen.
Reviewer's conclusions: There is not enough evidence to assess the equivalence of effect between fully intermittent, rifampicin-containing short-course chemotherapy and similar daily therapy in patients with pulmonary tuberculosis. Larger randomised studies are required to establish the effectiveness of fully intermittent, short-course chemotherapy.
Conflict of interest statement
We certify that we have no affiliations with or involvement in any organization or entity with a direct financial interest in the subject matter of the review (eg employment, consultancy, stock ownership, honoraria, expert testimony).
Figures





References
References to studies included in this review
Hong Kong 1981 {published data only}
-
- Hong Kong Chest Service/ British Medical Research Council. Controlled trial of four thrice weekly regimens and a daily regimen all given for 6 months for pulmonary tuberculosis. Lancet 1981;1:171‐4. - PubMed
References to studies excluded from this review
Brazil 1989 {published data only}
-
- Castelo A, Jardim JR, Goihman S, Kalckman AS, Dalboni MA, Silva EA, et al. Comparison of daily and twice‐weekly regimens to treat pulmonary tuberculosis. Lancet 1989;2:1173‐6. - PubMed
Hong Kong 1974 {published data only}
-
- Hong Kong Tuberculosis Treatment Services/Brompton Hospital/British Medical Research Council. A controlled clinical trial of daily and intermittent regimens of rifampicin plus ethambutol in the retreatment of patients with pulmonary tuberculosis in Hong Kong. Tubercle 1974;55:1‐27. - PubMed
Hong Kong 1982 {published data only}
-
- Hong Kong Chest Service/British Medical Research Council. Controlled trial of 4 three‐times‐weekly regimens and a daily regimen all given for 6 months for pulmonary tuberculosis. Second report: the results up to 24 months. Tubercle 1982;63:89‐98. - PubMed
Hong Kong 1987 {published data only}
-
- Hong Kong Chest Service/British Medical Research Council. Five‐year follow‐up of a controlled trial of five 6‐month regimens of chemotherapy for pulmonary tuberculosis. American Review of Respiratory Disease 1987;136:1339‐42. - PubMed
India 1990 {published data only}
-
- Kumar L, Dhand R, Singhi PD, Rao KLN, Katariya S. A randomized trial of fully intermittent vs. daily followed by intermittent short course chemotherapy for childhood tuberculosis. Paediatric Infectious Disease Journal 1990;9(11):802‐6. - PubMed
Korea 1988 {published data only}
-
- Hong YP, Kim SC, Chang SC, Kim SJ, Jin BW, Park CD. Comparison of a daily and three intermittent retreatment regimens for pulmonary tuberculosis administered under programme conditions. Tubercle 1988;69:241‐53. - PubMed
South Africa 2000 {published data only}
-
- Water Naude JM, Donald PR, Hussey GD, Kibel MA, Louw A, Perkins DR, et al. Twice weekly vs. daily chemotherapy for childhood tuberculosis. Pediatric Infectious Disease Journal 2000;19(5):405‐10. - PubMed
Additional references
Alwood 1994
-
- Alwood K, Keruly J, Moore‐Rice K, Stanton DL, Chaulk CP, Chaisson RE. Effectiveness of supervised, intermittent therapy for tuberculosis in HIV‐infected patients. AIDS 1994;8(8):1103‐8. - PubMed
Anonymous 1993
-
- Anonymous. Approaches to improving adherence to antituberculosis therapy‐‐South Carolina and New York, 1986‐1991. MMWR. Morbidy and Mortality Weekly Report 1993;42:74‐5,81. - PubMed
Bechan 1997
-
- Bechan S, Connolly C, Short GM, Standing E, Wilkinson D. Directly observed therapy for tuberculosis given twice weekly in the workplace in urban South Africa. Transactions of the Royal Society of Tropical Medicine and Hygiene 1997;91:704‐7. - PubMed
BTS 1984
-
- British Thoracic Society. A controlled trial of 6 months chemotherapy in pulmonary tuberculosis, final report : results during the 36 months after the end of chemotherapy and beyond. British Journal of Diseases of the Chest 1984;78:330‐6. - PubMed
Caminero 1996
Chaulk 1995
-
- Chaulk CP, Moore‐Rice K, Rizzo R, Chaisson RE. Eleven years of community‐based directly observed therapy for tuberculosis. Journal of the American Medical Association 1995;274(12):945‐51. - PubMed
China 1996
-
- China Tuberculosis Control Collaboration. Results of a directly observed short course chemotherapy in 112842 Chinese patients with smear positive tuberculosis. Lancet 1996;343:358‐62. - PubMed
Clarke 2002
-
- Clarke M, Oxman AD, editors. Optimal search strategy. Cochrane Reviewers' Handbook 4.1.5 [updated April 2002]; Appendix 5c. In: The Cochrane Library [database on disk and CDROM]. The Cochrane Collaboration. Oxford: Update Software; 2002, Issue 4.
Cohn 1990
-
- Cohn DL, Catlin BJ, Peterson KL, Judson FN, Sbarbaro JA. A 62‐dose, 6 months therapy for pulmonary and extrapulmonary tuberculosis: a twice‐weekly, directly observed and cost‐effective regimen. Annals of Internal Medicine 1990;112:407‐15. - PubMed
Crowle 1986
-
- Crowle AJ, Sbarbaro JA, May MH. Inhibition by pyrazinamide of tubercle bacilli within cultured human macrophages. American Review of Respiratory Disease 1986;134:1052‐5. - PubMed
Davies 1999
-
- Davies GR, Connolly C, Sturm AW, McAdam KP, Wilkinson D. Twice‐weekly, directly observed treatment for HIV‐infected and uninfected tuberculosis patients: cohort study in rural South Africa. AIDS 1999;13(7):811‐7. - PubMed
Dolin 1994
Dutt 1979
-
- Dutt AK, Jones L, Stead WW. Short‐course chemotherapy for tuberculosis with largely twice‐weekly isoniazid‐rifampin. Chest 1979;75:441‐7. - PubMed
E&C Africa 1983
-
- East & Central African/British Medical Research Councils' Fifth Collaborative Study. Controlled clinical trial of 4 short‐course regimens of chemotherapy (three 6‐month and one 8‐month) for pulmonary tuberculosis. Tubercle 1983;64:153‐66. - PubMed
Enarson 1996
-
- Enarson DA, Rieder HL, Arnadottir T, Trebucq A. Tuberculosis guide for low income countries. 4th Edition. Frankfurt: pmi Verlagsgruppe, 1996.
Grzybowski 1975
-
- Grzybowski S, Barnett GD, Styblo K. Contacts of cases of active pulmonary tuberculosis. Bulletin of the International Union Against Tuberculosis 1975;50:90‐106. - PubMed
Harries 1996
-
- Harries AD, Nyong'Onya Mbewe L, Salaniponi FM, Nyangulu DS, Veen J, Ringdal T, et al. Tuberculosis programme changes and treatment outcomes in patients with smear‐positive pulmonary tuberculosis in Blantyre, Malawi. Lancet 1996;347:807‐9. - PubMed
Hong Kong 1991a
-
- HongKong Chest Service / British Medical Research Council. Controlled trial of 2, 4, and 6 months of pyrazinamide in 6‐month, three‐times‐weekly regimens for smear‐positive pulmonary tuberculosis, including an assessment of a combined preparation of isoniazid, rifampin, and pyrazinamide. Am Rev Respir Dis 1991;143:700‐6. - PubMed
Hong Kong 1991b
-
- Hong Kong Chest Service / Tuberculosis Research Centre, Madras / British Medical Research Council. A controlled clinical comparison of 6 and 8 months of antituberculosis chemotherapy in the treatment of patients with silicotuberculosis in Hong Kong. American Review of Respiratory Disease 1991;143:262‐7. - PubMed
Manalo 1990
-
- Manalo F, Tan F, Sbarbaro JA, Iseman MD. Community‐based short‐course treatment of pulmonary tuberculosis in a developing nation. Initial report of an eight‐month, largely intermittent regimen in a population with a high prevalence of drug resistance. American Review of Respiratory Disease 1990;142:1301‐5. - PubMed
Neher 1996
-
- Neher A, Breyer G, Shrestha B, Feldmann K. Directly observed short‐course chemotherapy in the Kathmandu valley. Tubercle and Lung Disease 1996;77:302‐7. - PubMed
Roumania 1977
-
- Tuberculosis Research Institute, Bucharest. Trial of two intermittent short‐course regimens (78 doses) in the initial treatment of pulmonary tuberculosis. Tubercle 1977;58:1‐8. - PubMed
Sedlaczek 1995
-
- Sedlaczek AM, Serwatowski P, Spiewak W. Evaluation of the efficacy of treatment for smear‐positive pulmonary tuberculosis with early introduction of the interrupting method ‐ preliminary report. Pneumonologia i Alergologia Polska 1995;63:293‐7. - PubMed
Singapore 1981
-
- Singapore Tuberculosis Service/British Medical Research Council. Clinical trial of six‐month and four‐month regimens of chemotherapy in the treatment of pulmonary tuberculosis: the results up to 30 months. Tubercle 1981;62:95‐102. - PubMed
Weis 1994
-
- Weis SE, Slocum PC, Blais FX, King B, Nunn M, Matney GB, et al. The effect of directly observed therapy on the rates of drug resistance and relapse in tuberculosis. New England Journal of Medicine 1994;330:1179‐84. - PubMed
WHO 1996
-
- Global Tuberculosis Programme. Tuberculosis notification update. World Health Organization Weekly Epidemiological Record 1996;71(9):65‐72.
Wilkinson 1997a
-
- Wilkinson D, Floyd K, Gilks CF. Cost and cost‐effectiveness of alternative tuberculosis management strategies in South Africa‐‐implications for policy. South African Medical Journal 1997;87:451‐5. - PubMed
Wilkinson 1997b
-
- Wilkinson D, Davies GR. Coping with Africa's increasing tuberculosis burden: are community supervisors an essential component of the DOT strategy?. Tropical Medicine and International Health 1997;2(7):700‐4. - PubMed
Wilkinson 1997c
-
- Wilkinson D, Anderson E, Davies GR, Sturm AW, McAdam KP. Efficacy of twice weekly treatment for tuberculosis given under direct observation in Africa. Transactions of the Royal Society of Tropical Medicine and Hygiene 1997;91(1):87‐9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

