Maximal androgen blockade for advanced prostate cancer
- PMID: 10796804
- PMCID: PMC10759791
- DOI: 10.1002/14651858.CD001526
Maximal androgen blockade for advanced prostate cancer
Abstract
Objectives: This systematic review assessed the effect of maximal androgen blockade (MAB) on survival when compared to castration (medical or surgical) alone for patients with advanced prostate cancer.
Search strategy: Randomized controlled trials were searched in general and specialized databases (MEDLINE, EMBASE, Cancerlit, Cochrane Library, VA Cochrane Prostate Disease register) and by reviewing bibliographies.
Selection criteria: All published randomized trials were eligible for inclusion provided they (1) randomized men with advanced prostate cancer to receive a non-steroidal anti-androgen (NSAA) medication in addition to castration (medical or surgical) or to castration alone, and (2) reported overall survival, progression-free survival, cancer-specific survival, and/or adverse events. Eligibility was assessed by two independent reviewers.
Data collection and analysis: Information on patients, interventions, and outcomes were extracted by two independent reviewers using a standardized form. The main outcome measure for comparing effectiveness was overall survival at 1, 2, and 5 years. Secondary outcome measures included progression-free survival and cancer-specific survival. The relationship of specific NSAA on outcome was evaluated. Additionally, the incidence of adverse effects was measured.
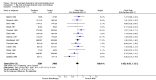
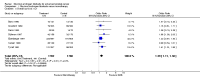
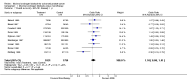
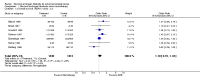
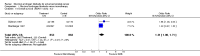
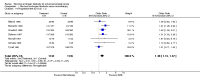
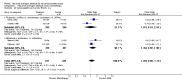
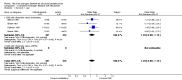
Main results: Twenty trials enrolling 6,320 patients were included. The pooled OR for overall survival was 1.03 (95% CI:0.85 to 1.25), 1.16 (95% CI:1.00 to 1.33), and 1.29 (95% CI:1.11 to 1.50) at 1, 2, and 5 years respectively. Overall survival was only significant at 5 years. The risk difference at 5 years was 0.048 (95% CI:0.02 to 0.077) and NNT at 5 years 20.8. Progression-free survival was improved only at 1 year follow-up (OR=1.38) and cancer-free survival was improved only at 5 years (OR=1.22). Adverse events occurred more frequently in those assigned to MAB and resulted in withdrawal in 10%. Quality of life was measured in only one study favored orchiectomy alone (less diarrhea and better emotional functioning in the first 6 months).
Reviewer's conclusions: MAB produces a modest overall and cancer-specific survival at 5 years but is associated with increased adverse events and reduced quality of life.
Conflict of interest statement
B Schmitt has co‐authored a draft manuscript with the Blue Cross/Blue Shield Technology Evaluation Center project on hormonal therapies for prostate cancer.
CL Bennett is (1) on the speaker's bureau for Schering‐Plough and ALZA; (2) has received unrestricted grant support from Schering‐Plough, AstraZeneca, and Cell Pathways; and (3) was a consultant to the Blue Cross/Blue Shield Technology Evaluation Center project on hormonal therapies for prostate cancer.
TJ Wilt was a consultant to the Blue Cross/Blue Shield Technology Evaluation Center project on hormonal therapies for prostate cancer.
Figures




























References
References to studies included in this review
Beland 1990 {published data only}
-
- *Beland G, Elhilali M, Fradet Y, Laroche B, Ramsey EW, Trachtenberg J, Venner PM, Tewari HD. A controlled trial of castration with and without nilutamide in metastatic prostatic carcinoma. Cancer 1990;66(5 Suppl):1074‐9. [MEDLINE: ] - PubMed
-
- Beland G. Combination of Anandron with orchiectomy in treatment of metastatic prostate cancer. Results of a double‐blind study. Urology 1991;37(2 Suppl):25‐9. [MEDLINE: ] - PubMed
-
- Beland G, Elhilali M, Fradet Y, Laroche B, Ramsey EW, Trachtenberg J, Venner PM. Total androgen blockade for metastatic cancer of the prostate. Am J Clin Oncol 1988;11(Suppl 2):S187‐90. [MEDLINE: ] - PubMed
-
- Beland G, Elhilali M, Fradet Y, Laroche B, Ramsey EW, Trachtenberg J, Venner PM, Tewari HD. A controlled trial of castration with and without nilutamide in metastatic prostatic carcinoma. Cancer 1990b;66(5 Suppl):1074‐9. [MEDLINE: ] - PubMed
-
- Beland G, Elhilali M, Fradet Y, Laroche B, Ramsey EW, Trachtenberg J, Venner PM, Tewari HD. Total androgen ablation: Canadian experience. Urol Clin North Am 1991;18(1):75‐82. [MEDLINE: ] - PubMed
Boccardo 1993 {published data only}
-
- Boccardo F, Pace M, Rubagotti A, Guarneri D, Decensi A, Oneto F, Martorana G, Giuliani L, Selvaggi F, Battaglia M, et al. Goserelin acetate with or without flutamide in the treatment of patients with locally advanced or metastatic prostate cancer. The Italian Prostatic Cancer Project (PONCAP) Study Group. Eur J Cancer 1993;29A(8):1088‐93. [MEDLINE: ] - PubMed
Bono 1998 {published data only}
-
- Bono AV, DiSilverio F, Robustelli della Cuna G, Benvenuti C, Brausi M, Ferrari P, Gibba A, Galli L. Complete androgen blockade versus chemical castration in advanced prostatic cancer: analysis of an Italian multicentre study. Italian Leuprorelin Group. Urol Int 1998;60(Suppl 1):18‐24. [MEDLINE: ] - PubMed
Brisset 1987 {published data only}
-
- Brisset JM, Boccon‐Gibod L, Botto H, Camey M, Cariou G, Duclos JM, Duval F, Gonties D, Jorest R, Lamy L, et al. Anandron (RU 23908) associated to surgical castration in previously untreated stage D prostate cancer: a multicenter comparative study of two doses of the drug and of a placebo. Prog Clin Biol Res 1987;243A:411‐22. [MEDLINE: ] - PubMed
Crawford 1989 {published data only}
-
- *Crawford ED, Eisenberger MA, McLeod DG, Spaulding JT, Benson R, Dorr FA, Blumenstein BA, Davis MA, Goodman PJ. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med 1989;321(7):419‐24. [MEDLINE: ] - PubMed
-
- Benson RC. Total androgen blockade: the United States experience. Eur Urol 1993;24(Suppl 2):72‐6. [MEDLINE: ] - PubMed
-
- Benson RC, Crawford ED, Eisenberger MA, McLeod DG, Spaulding JT, Dorr FA. National Cancer Institute study of luteinizing hormone‐releasing hormone plus flutamide versus luteinizing hormone‐releasing hormone plus placebo. Semin Oncol 1991;5(Suppl 6):9‐12. [MEDLINE: ] - PubMed
-
- Crawford ED. Changing concepts in the management of advanced prostate cancer. Urology 1995;46(6):899‐901. - PubMed
-
- Crawford ED. Combination studies with leuprolide. Eur Urol 1990;18(Suppl 3):30‐3. [MEDLINE: ] - PubMed
Crawford 1990 {published data only}
-
- Crawford ED, Kasimis BS, Gandara D, Smith JA, Soloway MS, Lange PH, Lynch DF, Al‐Juburi A, Bracken RB, Wise HA, et al. A randomized, controlled clinical trial of leuprolide and anandron (LA) vs leuprolide and placebo (LP) for advanced prostate cancer (D2cap) [abstract]. Proc Annu Meet Am Soc Clin Oncol 1990;9:A523.
Denis 1993 {published data only}
-
- *Denis LJ, Carnelro de Moura JL, Bono A, Sylvester R, Whelan P, Newling D, Depauw M. Goserelin acetate and flutamide versus bilateral orchiectomy: a phase III EORTC trial (30853). EORTC GU Group and EORTC Data Center. Urology 1993;42(2):119‐29. [MEDLINE: ] - PubMed
-
- Carvalho AP, Moura JL, Denis L, Newling D, Smith P, Bono A, Sylvester R, Pauw M, Ongena P. Zoladex and flutamide vs orchidectomy: a phase III EORTC 30853 trial. EORTC Urological Group. Prog Clin Biol Res 1989;303:129‐43. - PubMed
-
- Denis L, Keuppens F, Newling D, Smith PH, Calais da Silva F, Sylvester R, DePauw M, Onegana P and members of the EORTC‐GU Group Belgium. Orchidectomy versus total androgen blockade: a phase III EORTC 30853 study. GnRH Analogues Cancer Human Reproduction 1990;3:117‐27.
-
- Denis L, Keuppens F, Robinson M, Mahler C, Smith P, Pinto de Carvalho AP, Newling D, Bono A, Sylvester R, Pauw M, et al. Complete androgen blockade: data from an EORTC 30853 trial. Semin Urol 1990;8(3):166‐74. - PubMed
-
- Denis L, Robinson M, Mahler C, Smith P, Keuppens F, Moura JL, Bono A, Newling D, Sylvester R, Pauw M, et al. Orchidectomy versus Zoladex plus Eulexin in patients with metastatic prostate cancer (EORTC 30853). J Steroid Biochem Mol Biol 1990;37(6):951‐9. - PubMed
Dijkman 1997 {published data only}
-
- *Dijkman GA, Janknegt RA, Reijke TM, Debruyne FM. Long‐term efficacy and safety of nilutamide plus castration in advanced prostate cancer, and the significance of early prostate specific antigen normalization. International Anandron Study Group. J Urol 1997;158(1):160‐3. [MEDLINE: ] - PubMed
-
- Dijkman GA, Fernandez del Moral P, Debruyne FM, Janknegt RA. Improved subjective responses to orchiectomy plus nilutamide (Anandron) in comparison to orchiectomy plus placebo in metastatic prostate cancer. International Anandron Study Group. Eur Urol 1995;27(3):196‐201. - PubMed
-
- Janknegt RA. Total androgen blockade with the use of orchiectomy and nilutamide (Anandron) or placebo as treatment of metastatic prostate cancer. International Anandron Study Group. Cancer 1993;72(12 Suppl):3874‐7. - PubMed
-
- Janknegt RA, Abbou CC, Bartoletti R, Bernstein‐Hahn L, Bracken B, Brisset JM, Silva FC, Chisholm G, Crawford ED, Debruyne FM, et al. Orchiectomy and nilutamide or placebo as treatment of metastatic prostate cancer in a multinational double‐blind randomized trial. J Urol 1993;149(1):77‐82. - PubMed
Eisenberger 1997 {published and unpublished data}
-
- *Eisenberger M, Blumenstein B, Crawford ED, Miller G, McLeod D, Loehrer P, Wilding G, Sears K, Culkin DJ, Thompson IM, Bueschen AJ, Lowe BA. Bilateral orchidectomy with or without flutamide for metastatic prostate cancer. N Engl J Med 1998;339:1036‐42. [MEDLINE: ] - PubMed
-
- Eisenberger M, Crawford ED, McLeod D, Loehrer P, Wilding G, Blumenstein B. A comparison of bilateral orchidectomy (orch) with or without flutamide in stage D2 prostate cancer (PC) (NCI INT‐0105 SWOG/ECOG). Proc Annu Meet AM Soc Clin Oncol 1997;16:A3.
Ferrari 1993 {published data only}
-
- Ferrari P, Castagnetti G, Ferrari G, Pollastri CA, Tavoni F, Dotti A. Combination treatment in M1 prostate cancer. Cancer 1993;72(12 Suppl):3880‐5. [MEDLINE: ] - PubMed
Ferrari 1996 {published data only}
-
- Ferrari P, Castagnetti G, Ferrari G, Baisi B, Dotti A. Combination treatment versus LHRH alone in advanced prostatic cancer. Urol Int 1996;56(Suppl 1):13‐7. [MEDLINE: ] - PubMed
Fourcade 1990 {published data only}
-
- Fourcade RO, Cariou G, Coloby P, Colombel P, Coulange C, Grise P, Mangin P, Soret JY, Poterre M. Total androgen blockade with Zoladex plus flutamide vs. Zoladex alone in advanced prostatic carcinoma: interim report of a multicenter, double‐blind, placebo‐controlled study. Eur Urol 1990;18(Suppl 3):45‐7. [MEDLINE: ] - PubMed
Iversen 1990 {published data only}
-
- *Iversen P, Christensen MG, Friis E, Hornbol P, Hvidt V, Iversen HG, Klarskov P, Krarup T, Lund F, Mogensen P, et, al. A phase III trial of zoladex and flutamide versus orchiectomy in the treatment of patients with advanced carcinoma of the prostate. Cancer 1990;66(5 Suppl):1058‐66. [MEDLINE: ] - PubMed
-
- Iversen P. Zoladex plus flutamide vs. orchidectomy for advanced prostatic cancer. Danish Prostatic Cancer Group (DAPROCA). Eur Urol 1990;18(Suppl 3):41‐4. [MEDLINE: ] - PubMed
-
- Iversen P, Rasmussen F, Klarskov P, Christensen IJ. Long‐term results of Danish Prostatic Cancer Group trial 86. Goserelin acetate plus flutamide versus orchiectomy in advanced prostate cancer. Cancer 1993;72(12 Suppl):3851‐4. [MEDLINE: ] - PubMed
Knonagel 1989 {published data only}
-
- Knonagel H, Bolle JF, Hering F, Senn E, Hodel T, Neuenschwander H, Biedermann C. Therapy of metastatic prostatic cancer by orchiectomy plus Anandron versus orchiectomy plus placebo. Initial results of a randomized multicenter study. Helv Chir Acta 1989;56(3):343‐5. [MEDLINE: ] - PubMed
Namer 1990 {published data only}
-
- *Namer M, Toubol J, Caty A, Couette JE, Douchez J, Kerbrat P, Droz JP. A randomized double‐blind study evaluating Anandron associated with orchiectomy in stage D prostate cancer. J Steroid Biochem Mol Biol 1990;37(6):909‐15. [MEDLINE: ] - PubMed
-
- Namer M, Amiel J, Toubal J. Anandron (RU 23908) associated with orchiectomy in stage D prostate cancer. Preliminary results of a randomized, double‐blind study. Am J Clin Oncol 1988;11(Suppl 2):S191‐6. - PubMed
Navratil 1987 {published data only}
-
- Navratil H. Double‐blind study of Anandron versus placebo in stage D2 prostate cancer patients receiving buserelin. Results on 49 cases from a multicentre study. Prog Clin Biol Res 1987;243A:401‐10. [MEDLINE: ] - PubMed
Periti 1995 {published data only}
-
- Periti P, Rizzo M, Mazzei T, Mini E. Depot leuprorelin acetate alone or with nilutamide in the treatment of metastatic prostate carcinoma: interim report of a multicenter, double‐blind, placebo‐controlled study [abstract]. Can J Infect 1995;6(Suppl C):292C.
Schulze 1988 {published data only}
-
- Schulze H, Kaldenhoff H, Senge T. Evaluation of total versus partial androgen blockade in the treatment of advanced prostatic cancer. Urol Int 1988;43(4):193‐7. [MEDLINE: ] - PubMed
Tyrrell 1991 {published data only}
-
- *Tyrrell CJ, Altwein JE, Klippel F, Varenhorst E, Lunglmayr G, Boccardo F, Holdaway IM, Haefliger JM, Jordaan JP. A multicenter randomized trial comparing the luteinizing hormone‐releasing hormone analogue goserelin acetate alone and with flutamide in the treatment of advanced prostate cancer. The International Prostate Cancer Study Group. J Urol 1991;146(5):1321‐6. [MEDLINE: ] - PubMed
-
- Jurincic CD, Horlbeck R, Klippel KF. Combined treatment (goserelin plus flutamide) versus monotherapy (goserelin alone) in advanced prostate cancer: a randomized study. Semin Oncol 1991;18(5 Suppl 6):21‐5. [MEDLINE: ] - PubMed
-
- Jurincic CD, Winkler C, Horlbeck R, Gasser A, Klippel KF. The treatment of advanced prostatic carcinoma with goserelin acetate (Zoladex (r)) and goserelin acetate plus (Fugerel (r)). Dtsch Z Onkol 1992;24(3):65‐70.
-
- Lunglmayr G. 'Zoladex' versus 'Zoladex' plus flutamide in the treatment of advanced prostate cancer. First interim analysis of an international trial. International Prostate Cancer Study Group. Prog Clin Biol Res 1989;303:145‐51. [MEDLINE: ] - PubMed
-
- Tyrrell CJ, Altwein JE, Klippel F, Varenhorst E, Lunglmayr G, Boccardo F, Holdaway IM, Haefliger JM, Jordaan JP, Sotarauta M. Multicenter randomized trial comparing Zoladex with Zoladex plus flutamide in the treatment of advanced prostate cancer. Survival update. International Prostate Cancer Study Group. Cancer 1993;72(12 Suppl):3878‐9. [MEDLINE: ] - PubMed
Zalcberg 1996 {published data only}
-
- Zalcberg JR, Raghaven D, Marshall V, Thompson PJ. Bilateral orchidectomy and flutamide versus orchidectomy alone in newly diagnosed paptients with metastatic carcinoma of the prostate‐an Australian multicentre trial. Br J Urol 1996;77(6):865‐9. [MEDLINE: ] - PubMed
References to studies excluded from this review
de Voogt 1990 {published data only}
-
- Voogt HJ, Klijn JG, Studer U, Schroder F, Sylvester R, Pauw M. Orchidectomy versus Buserelin in combination with cyproterone acetate, for 2 weeks or continuously, in the treatment of metastatic prostatic cancer. Preliminary results of EORTC‐trial 30843. J Steroid Biochem Mol Biol 1990;37(6):965‐9. [MEDLINE: ] - PubMed
Di Silverio 1990 {published data only}
-
- Silverio F, Serio M. [Zoladex in prostatic carcinoma]. Drugs Exp Clin Res 1990;16(Suppl):19‐29. [MEDLINE: ] - PubMed
-
- Silverio F, Serio M, D'Eramo G, Sciarra F. Zoladex vs. Zoladex plus cyproterone acetate in the treatment of advanced prostatic cancer: a multicenter Italian study. Eur Urol 1990;18(Suppl 3):54‐61. - PubMed
Jorgensen 1993 {published data only}
-
- Jorgensen T, Tveter KJ, Jorgensen LH. Total androgen suppression: experience from the Scandinavian Prostatic Cancer Group Study No. 2. Eur Urol 1993;24(4):466‐70. [MEDLINE: ] - PubMed
Klosterhalfen 1987 {published data only}
-
- Klosterhalfen H, Becker H. Results of a 10‐year randomised prospective study in metastasised prostate cancer. Aktuel Urol 1987;18(5):234‐6.
Robinson 1995 {published data only}
-
- Newling DW, Pavone Macaluse M, Smith P, Voogt HJ, Robinson MR, Schroder FH, Denis L, Jones WG, Pauw M, Sylvester R. Update of EORTC clinical trials in prostate cancer. The EORTC Genito‐Urinary Group. Semin Urol 1992;10(1). - PubMed
-
- Robinson MR. A further analysis of European Organization for Research and Treatment of Cancer protocol 30805. Orchidectomy versus orchidectomy plus cyproterone acetate versus low‐dose diethylstilbestrol. Cancer 1993;72(12 Suppl). [MEDLINE: ] - PubMed
-
- Robinson MR. Complete androgen blockade: the EORTC experience comparing orchidectomy versus orchidectomy plus cyproterone acetate versus low‐dose stilboestrol in the treatment of metastatic carcinoma of the prostate. Prog Clin Biol Res 1987;243A:383‐90. - PubMed
-
- Robinson MR. EORTC protocol 30805: a phase III trial comparing orchidectomy versus orchidectomy and cyproterone acetate and low dose stilboestrol in the management of metastatic carcinoma of the prostate. Prog Clin Biol Res 1988;260:101‐10. - PubMed
-
- Robinson MR, Smith PH, Richards B, Newling DW, Pauw M, Sylvester R. The final analysis of the EORTC Genito‐Urinary Tract Cancer Co‐Operative Group phase III clinical trial (protocol 30805) comparing orchidectomy, orchidectomy plus cyproterone acetate and low dose stilboestrol in the management of metastatic carcinoma of the prostate. Eur Urol 1995;28(4):273‐83. - PubMed
Thorpe 1996 {published data only}
-
- Thorpe SC, Azmatullah S, Fellows GJ, Gingell JC, O'Boyle PJ. A prospective, randomised study to compare goserelin acetate (Zoladex) versus cyproterone acetate (Cyprostat) versus a combination of the two in the treatment of metastatic prostatic carcinoma. Eur Urol 1996;29(1):47‐54. [MEDLINE: ] - PubMed
Williams 1990 {published data only}
-
- Williams G, Asopa R, Abel PD, Smith C. Pituitary adrenal and gonadal endocrine suppression for the primary treatment of prostate cancer. Br J Urol 1990;65(5):504‐8. [MEDLINE: ] - PubMed
Additional references
Bennett 1999
-
- Bennett CL, Tosteson T, Weinberg P, et al. Maximum androgen blockade with flutamide in conjunction with surgical or medical castration therapy for metastatic prostate cancer: a literature‐based meta‐analysis of 9 randomized trials and 4, 128 patients. Prostate cancer and prostatic diseases. Prostate Cancer Prostatic Dis 1999;2:4‐8. - PubMed
Conn 1991
-
- Conn PM, Crowley WF Jr. Gonadotropin‐releasing hormone and its analogue. N Engl J Med 1991;324:93‐103. - PubMed
Crawford 1989
-
- Crawford ED, Eisenberger MA, McLeod DG, Spaulding JT, Benson R, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med 1989;321(7):419‐24. [MEDLINE: ] - PubMed
Denis 1993a
-
- Denis LJ, Carnelro de Moura JL, Bono A, Sylvester R, Whelan P, Newling D, Depauw M. Goserelin acetate and flutamide versus orchiectomy: a phase III EORTC trial (30853). Urology 1993;42(2):119‐30. [MEDLINE: ] - PubMed
Denis 1993b
-
- Denis L, Murphy GP. Overview of phase III trials on combined androgen treatment in patients with metastatic prostate cancer. Cancer 1993;72(12 Suppl):3888‐95. [MEDLINE: ] - PubMed
Hasselblad 1998
-
- Hasselblad V. Meta‐analysis of multitreatment studies. Med Decision Making 1998;18(1):37‐43. - PubMed
Landis 1999
-
- Landis SH, Murray T, Bolden S, Wingo PA. Cancer Statistics, 1999. A Cancer Journal for Clinicians 1999;49:8‐11. - PubMed
Moinpour 1998
-
- Moinpour CM, Savage M, Troxel A, Lovato L, et al. Quality of life in advanced prostate cancer: results of a randomized therapeutic trial. J Natl Cancer Inst 1998;90:1537‐44. - PubMed
PCTOG 1995
-
- Prostate Cancer Trialists' Overview Group. Maximum androgen blockade in advanced prostate cancer: An overview of 22 randomized trials with 3283 deaths in 5710 patients. Lancet 1995;346:265‐69. [MEDLINE: ] - PubMed
References to other published versions of this review
Aronson 1999
-
- Aronson N, Seidenfeld J, Samson DJ, et al. Relative Effectiveness and Cost‐Effectiveness of Methods of Androgen Suppression in the Treatment of Advanced Prostate Cancer. Evidence Report/Technology Assessment No. 4. (Prepared by Blue Cross/Blue Shield Association Evidence‐based Practice Center under Contract No. 290‐97‐0015). AHCPR Publication No. 99‐E0012. Rockville, MD: Agency for Health Care Policy and Research, May 1999. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

