Multisite comparison of CD4 and CD8 T-lymphocyte counting by single- versus multiple-platform methodologies: evaluation of Beckman Coulter flow-count fluorospheres and the tetraONE system. The NIAID DAIDS New Technologies Evaluation Group
- PMID: 10799444
- PMCID: PMC95877
- DOI: 10.1128/CDLI.7.3.344-351.2000
Multisite comparison of CD4 and CD8 T-lymphocyte counting by single- versus multiple-platform methodologies: evaluation of Beckman Coulter flow-count fluorospheres and the tetraONE system. The NIAID DAIDS New Technologies Evaluation Group
Abstract
New analytic methods that permit absolute CD4 and CD8 T-cell determinations to be performed entirely on the flow cytometer have the potential for improving assay precision and accuracy. In a multisite trial, we compared two different single-platform assay methods with a predicate two-color assay in which the absolute lymphocyte count was derived by conventional hematology. A two-color method employing lymphocyte light scatter gating and Beckman Coulter Flow-Count fluorospheres for absolute counting produced within-laboratory precision equivalent to that of the two-color predicate method, as measured by coefficient of variation of replicate measurements. The fully automated Beckman Coulter tetraONE System four-color assay employing CD45 lymphocyte gating, automated analysis, and absolute counting by fluorospheres resulted in a small but significant improvement in the within-laboratory precision of CD4 and CD8 cell counts and percentages suggesting that the CD45 lymphocyte gating and automated analysis might have contributed to the improved performance. Both the two-color method employing Flow-Count fluorospheres and the four-color tetraONE System provided significant and substantial improvements in between-laboratory precision of absolute counts. In some laboratories, absolute counts obtained by the single-platform methods showed small but consistent differences relative to the predicate method. Comparison of each laboratory's absolute counts with the five-laboratory median value suggested that these differences resulted from a bias in the absolute lymphocyte count obtained from the hematology instrument in some laboratories. These results demonstrate the potential for single-platform assay methods to improve within-laboratory and between-laboratory precision of CD4 and CD8 T-cell determinations compared with conventional assay methods.
Figures

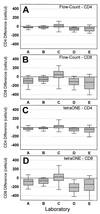
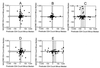
Comment in
-
Adoption of single-platform technologies for enumeration of absolute T-lymphocyte subsets in peripheral blood.Clin Diagn Lab Immunol. 2000 May;7(3):333-5. doi: 10.1128/CDLI.7.3.333-335.2000. Clin Diagn Lab Immunol. 2000. PMID: 10799442 Free PMC article. Review. No abstract available.
References
-
- Aboulker J P, Autran B, Beldjord K, Touraine F, Debre P. Consistency of routine measurements of CD4+, CD8+ peripheral blood lymphocytes. J Immunol Methods. 1992;154:155–161. - PubMed
-
- Calvelli T, Denny T N, Paxton H, Gelman R, Kagan J. Guideline for flow cytometric immunophenotyping: a report from the National Institute of Allergy and Infectious Diseases, Division of AIDS. Cytometry. 1993;14:702–715. - PubMed
-
- Centers for Disease Control and Prevention. 1997 revised guidelines for performing CD4+ T-cell determinations in persons infected with human immunodeficiency virus (HIV) Morbid Mortal Weekly Rep. 1997;46(RR-2):1–29. - PubMed
-
- Connelly M C, Knight M, Giorgi J V, Kagan J, Landay A L, Parker J W, Page E, Spino C, Wilkening C, Mercolino T J. Standardization of absolute CD4+ lymphocyte counts across laboratories: an evaluation of the Ortho CytoronAbsolute Flow Cytometry System on normal donors. Cytometry. 1995;22:200–210. - PubMed
-
- Giorgi J V, Cheng H-L, Margolick J B, Bauer K D, Ferbas J, Waxdal M, Schmid I, Hultin L E, Jackson A L, Park L, Taylor J M G the Multicenter AIDS Cohort Study Group. Quality control in the flow cytometric measurement of T-lymphocyte subsets: the Multicenter AIDS Cohort Study experience. Clin Immunol Immunopathol. 1990;55:173–186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

