Synergistic action of GA-binding protein and glucocorticoid receptor in transcription from the mouse mammary tumor virus promoter
- PMID: 10799572
- PMCID: PMC110850
- DOI: 10.1128/jvi.74.11.4988-4998.2000
Synergistic action of GA-binding protein and glucocorticoid receptor in transcription from the mouse mammary tumor virus promoter
Abstract
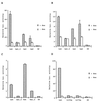
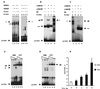
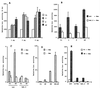
B lymphocytes are among the first cells to be infected by mouse mammary tumor virus (MMTV), and they play a crucial role in its life cycle. To study transcriptional regulation of MMTV in B cells, we have analyzed two areas of the long terminal repeat (LTR) next to the glucocorticoid receptor binding site, fp1 (at position -139 to -146 from the cap site) and fp2 (at -157 to -164). Both showed B-cell-specific protection in DNase I in vitro footprinting assays and contain binding sites for Ets transcription factors, a large family of proteins involved in cell proliferation and differentiation and oncogenic transformation. In gel retardation assays, fp1 and fp2 bound the heterodimeric Ets factor GA-binding protein (GABP) present in B-cell nuclear extracts, which was identified by various criteria: formation of dimers and tetramers, sensitivity to pro-oxidant conditions, inhibition of binding by specific antisera, and comigration of complexes with those formed by recombinant GABP. Mutations which prevented complex formation in vitro abolished glucocorticoid-stimulated transcription from an MMTV LTR linked to a reporter gene in transiently transfected B-cell lines, whereas they did not affect the basal level. Exogenously expressed GABP resulted in an increased level of hormone response of the LTR reporter plasmid and produced a synergistic effect with the coexpressed glucocorticoid receptor, indicating cooperation between the two. This is the first example of GABP cooperation with a steroid receptor, providing the opportunity for studying the integration of their intracellular signaling pathways.
Figures



References
-
- Acha-Orbea H, MacDonald H R. Superantigens of mouse mammary tumor virus. Annu Rev Immunol. 1995;13:459–486. - PubMed
-
- Basuyaux J P, Ferreira E, Stehelin D, Butticè G. The Ets transcription factors interact with each other and with the c-Fos/c-Jun complex via distinct protein domains in a DNA-dependent and -independent manner. J Biol Chem. 1997;272:26188–26195. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

