A DNA transfection system for generation of influenza A virus from eight plasmids
- PMID: 10801978
- PMCID: PMC18566
- DOI: 10.1073/pnas.100133697
A DNA transfection system for generation of influenza A virus from eight plasmids
Abstract
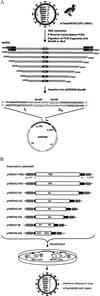
We have developed an eight-plasmid DNA transfection system for the rescue of infectious influenza A virus from cloned cDNA. In this plasmid-based expression system, viral cDNA is inserted between the RNA polymerase I (pol I) promoter and terminator sequences. This entire pol I transcription unit is flanked by an RNA polymerase II (pol II) promoter and a polyadenylation site. The orientation of the two transcription units allows the synthesis of negative-sense viral RNA and positive-sense mRNA from one viral cDNA template. This pol I-pol II system starts with the initiation of transcription of the two cellular RNA polymerase enzymes from their own promoters, presumably in different compartments of the nucleus. The interaction of all molecules derived from the cellular and viral transcription and translation machinery results in the generation of infectious influenza A virus. The utility of this system is proved by the recovery of the two influenza A viruses: A/WSN/33 (H1N1) and A/Teal/HK/W312/97 (H6N1). Seventy-two hours after the transfection of eight expression plasmids into cocultured 293T and MDCK cells, the virus yield in the supernatant of the transfected cells was between 2 x 10(5) and 2 x 10(7) infectious viruses per milliliter. We also used this eight-plasmid system for the generation of single and quadruple reassortant viruses between A/Teal/HK/W312/97 (H6N1) and A/WSN/33 (H1N1). Because the pol I-pol II system facilitates the design and recovery of both recombinant and reassortant influenza A viruses, it may also be applicable to the recovery of other RNA viruses entirely from cloned cDNA.
Figures


References
-
- Scholtissek C, Bürger H, Kistner O, Shortridge K F. Virology. 1985;147:287–294. - PubMed
-
- Claas E C, Osterhaus A D, van Beek R, De Jong J C, Rimmelzwaan G F, Senne D A, Krauss S, Shortridge K F, Webster R G. Lancet. 1998;351:472–477. - PubMed
-
- Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, Perdue M, Swayne D, Bender C, Huang J, et al. Science. 1998;279:393–396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

