Increased metalloproteinase activity, oxidant production, and emphysema in surfactant protein D gene-inactivated mice
- PMID: 10801980
- PMCID: PMC18543
- DOI: 10.1073/pnas.100448997
Increased metalloproteinase activity, oxidant production, and emphysema in surfactant protein D gene-inactivated mice
Abstract
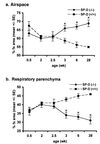
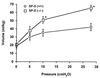
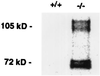
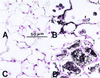
Targeted ablation of the surfactant protein D (SP-D) gene caused chronic inflammation, emphysema, and fibrosis in the lungs of SP-D (-/-) mice. Although lung morphology was unperturbed during the first 2 weeks of life, airspace enlargement was observed by 3 weeks and progressed with advancing age. Inflammation consisted of hypertrophic alveolar macrophages and peribronchiolar-perivascular monocytic infiltrates. These abnormalities were associated with increased activity of the matrix metalloproteinases, MMP2 and MMP9, and immunostaining for MMP9 and MMP12 in alveolar macrophages. Hydrogen peroxide production by isolated alveolar macrophages also was increased significantly (10-fold). SP-D plays a critical role in the suppression of alveolar macrophage activation, which may contribute to the pathogenesis of chronic inflammation and emphysema.
Figures




References
-
- Crouch E C. Biochim Biophys Acta. 1998;1408:278–289. - PubMed
-
- Reid K B M. Biochem Biophys Acta. 1998;1408:290–295. - PubMed
-
- Mason R J, Greene K, Voelker D R. Am J Physiol. 1998;275:L1–L13. - PubMed
-
- Crouch E, Rust K, Marienchek W, Parghi D, Chang D, Persson A. Am J Respir Cell Mol Biol. 1991;5:13–18. - PubMed
-
- Fisher J G, Mason R. Am J Respir Cell Mol Biol. 1995;12:13–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

