Functions of the nucleus of the optic tract (NOT). II. Control of ocular pursuit
- PMID: 10803412
- PMCID: PMC2002478
- DOI: 10.1007/s002219900302
Functions of the nucleus of the optic tract (NOT). II. Control of ocular pursuit
Abstract
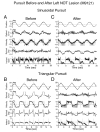
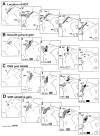
Ocular pursuit in monkeys, elicited by sinusoidal and triangular (constant velocity) stimuli, was studied before and after lesions of the nucleus of the optic tract (NOT). Before NOT lesions, pursuit gains (eye velocity/target velocity) were close to unity for sinusoidal and constant-velocity stimuli at frequencies up to 1 Hz. In this range, retinal slip was less than 2 degrees. Electrode tracks made to identify the location of NOT caused deficits in ipsilateral pursuit, which later recovered. Small electrolytic lesions of NOT reduced ipsilateral pursuit gains to below 0.5 in all tested conditions. Pursuit was better, however, when the eyes moved from the contralateral side toward the center (centripetal pursuit) than from the center ipsilaterally (centrifugal pursuit), although the eyes remained in close proximity to the target with saccadic tracking. Effects of lesions on ipsilateral pursuit were not permanent, and pursuit gains had generally recovered to 60-80% of baseline after about 2 weeks. One animal had bilateral NOT lesions and lost pursuit for 4 days. Thereafter, it had a centrifugal pursuit deficit that lasted for more than 2 months. Vertical pursuit and visually guided saccades were not affected by the bilateral NOT lesions in this animal. We also compared effects of these and similar NOT lesions on optokinetic nystagmus (OKN) and optokinetic after-nystagmus (OKAN). Correlation of functional deficits with NOT lesions from this and previous studies showed that rostral lesions of NOT in and around the pretectal olivary nucleus, which interrupted cortical input through the brachium of the superior colliculus (BSC), affected both smooth pursuit and OKN. In two animals in which it was tested, NOT lesions that caused a deficit in pursuit also decreased the rapid and slow components of OKN slow-phase velocity and affected OKAN. It was previously shown that slightly more caudal NOT lesions were more effective in altering gain adaptation of the angular vestibulo-ocular reflex (aVOR). The present findings suggest that cortical pathways through rostral NOT play an important role in maintenance of ipsilateral ocular pursuit. Since lesions that affected ocular pursuit had similar effects on ipsilateral OKN, processing for these two functions is probably closely linked in NOT, as it is elsewhere.
Figures







Similar articles
-
Eye movement deficits following ibotenic acid lesions of the nucleus prepositus hypoglossi in monkeys II. Pursuit, vestibular, and optokinetic responses.J Neurophysiol. 1999 Feb;81(2):668-81. doi: 10.1152/jn.1999.81.2.668. J Neurophysiol. 1999. PMID: 10036269
-
Functions of the nucleus of the optic tract (NOT). I. Adaptation of the gain of the horizontal vestibulo-ocular reflex.Exp Brain Res. 2000 Apr;131(4):416-32. doi: 10.1007/s002219900303. Exp Brain Res. 2000. PMID: 10803411
-
Effects of lesions of the nucleus of the optic tract on optokinetic nystagmus and after-nystagmus in the monkey.Exp Brain Res. 1990;79(2):225-39. doi: 10.1007/BF00608231. Exp Brain Res. 1990. PMID: 2323371
-
Contribution of the nucleus of the optic tract to optokinetic nystagmus and optokinetic afternystagmus in the monkey: clinical implications.Res Publ Assoc Res Nerv Ment Dis. 1990;67:233-55. Res Publ Assoc Res Nerv Ment Dis. 1990. PMID: 2106153 Review.
-
The nucleus of the optic tract. Its function in gaze stabilization and control of visual-vestibular interaction.Ann N Y Acad Sci. 1992 May 22;656:277-96. doi: 10.1111/j.1749-6632.1992.tb25215.x. Ann N Y Acad Sci. 1992. PMID: 1599149 Review.
Cited by
-
Whole-Brain Functional Ultrasound Imaging Reveals Brain Modules for Visuomotor Integration.Neuron. 2018 Dec 5;100(5):1241-1251.e7. doi: 10.1016/j.neuron.2018.11.031. Neuron. 2018. PMID: 30521779 Free PMC article.
-
Readaptation of the vestibulo-ocular reflex relieves the mal de debarquement syndrome.Front Neurol. 2014 Jul 15;5:124. doi: 10.3389/fneur.2014.00124. eCollection 2014. Front Neurol. 2014. PMID: 25076935 Free PMC article.
-
Electric field stimulation directs target-specific axon regeneration and partial restoration of vision after optic nerve crush injury.PLoS One. 2025 Jan 9;20(1):e0315562. doi: 10.1371/journal.pone.0315562. eCollection 2025. PLoS One. 2025. PMID: 39787061 Free PMC article.
-
Coding of Velocity Storage in the Vestibular Nuclei.Front Neurol. 2017 Aug 16;8:386. doi: 10.3389/fneur.2017.00386. eCollection 2017. Front Neurol. 2017. PMID: 28861030 Free PMC article.
-
Similar effects of feature-based attention on motion perception and pursuit eye movements at different levels of awareness.J Neurosci. 2012 May 30;32(22):7594-601. doi: 10.1523/JNEUROSCI.0355-12.2012. J Neurosci. 2012. PMID: 22649238 Free PMC article.
References
-
- Albright TD. Direction and orientation selectivity of neurons in visual area MT of the macaque. J Neurophysiol. 1984;52:1106–1130. - PubMed
-
- Brodal P. The corticopontine projection in the rhesus monkey. Origin and principles of organization. Brain. 1978a;101:251–283. - PubMed
-
- Brodal P. Principles of organization of the monkey corticopontine projection. Brain Res. 1978b;148:214–218. - PubMed
-
- Brodal P. Further observations on the cerebellar projections from the pontine nuclei and the nucleus reticularis tegmenti pontis in the rhesus monkey. J Comp Neurol. 1982;204:44–55. - PubMed
-
- Büttner U. The role of the cerebellum in smooth pursuit eye movements and optokinetic nystagmus in primates. Rev Neurol (Paris) 1989;145:560–566. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

