Hypoxia-induced silencing of NMDA receptors in turtle neurons
- PMID: 10804192
- PMCID: PMC6772670
- DOI: 10.1523/JNEUROSCI.20-10-03522.2000
Hypoxia-induced silencing of NMDA receptors in turtle neurons
Abstract
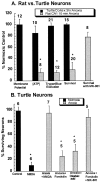
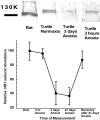
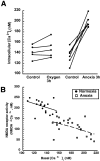
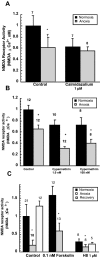
Hypoxia-induced suppression of NMDA receptors (NMDARs) in western painted turtle (Chrysemys picta) cortical neurons may be critical for surviving months of anoxic dormancy. We report that NMDARs are silenced by at least three different mechanisms operating at different times during anoxia. In pyramidal neurons from cerebrocortex, 1-8 min anoxia suppressed NMDAR activity (Ca(2+) influx and open probability) by 50-60%. This rapid decrease in receptor activity was controlled by activation of phosphatase 1 or 2A but was not associated with an increase in [Ca(2+)](i). However, during 2 hr of anoxia, [Ca(2+)](i) in cerebrocortical neurons increased by 35%, and suppression of NMDARs was predicted by the increase of [Ca(2+)](i) and controlled by calmodulin. An additional mechanism of NMDAR silencing, reversible removal of receptors from the cell membrane, was found in cerebrocortex of turtles remaining anoxic at 3 degrees C for 3-21 d. When suppression of NMDARs was prevented with phosphatase inhibitors, tolerance of anoxia was lost. Silencing of NMDARs is thus critical to the remarkable ability of C. picta to tolerate life without oxygen.
Figures


Similar articles
-
Reduction of NMDA receptor activity in cerebrocortex of turtles (Chrysemys picta) during 6 wk of anoxia.Am J Physiol. 1998 Jul;275(1):R86-91. doi: 10.1152/ajpregu.1998.275.1.R86. Am J Physiol. 1998. PMID: 9688964
-
Effect of anoxia and pharmacological anoxia on whole-cell NMDA receptor currents in cortical neurons from the western painted turtle.Physiol Biochem Zool. 2003 Jan-Feb;76(1):41-51. doi: 10.1086/374274. Physiol Biochem Zool. 2003. PMID: 12695985
-
delta-Opioid receptor antagonism induces NMDA receptor-dependent excitotoxicity in anoxic turtle cortex.J Exp Biol. 2008 Nov;211(Pt 21):3512-7. doi: 10.1242/jeb.021949. J Exp Biol. 2008. PMID: 18931323
-
Oxygen sensitive synaptic neurotransmission in anoxia-tolerant turtle cerebrocortex.Adv Exp Med Biol. 2012;758:71-9. doi: 10.1007/978-94-007-4584-1_10. Adv Exp Med Biol. 2012. PMID: 23080145 Review.
-
Anoxia and ischemia tolerance in turtle hearts.Braz J Med Biol Res. 1995 Nov-Dec;28(11-12):1233-40. Braz J Med Biol Res. 1995. PMID: 8728853 Review.
Cited by
-
Alleviating brain stress: what alternative animal models have revealed about therapeutic targets for hypoxia and anoxia.Future Neurol. 2013;8(3):287-301. doi: 10.2217/fnl.13.12. Future Neurol. 2013. PMID: 25264428 Free PMC article.
-
VEGF and Bcl-2 interact via MAPKs signaling pathway in the response to hypoxia in neuroblastoma.Cell Mol Neurobiol. 2009 May;29(3):391-401. doi: 10.1007/s10571-008-9331-9. Epub 2008 Dec 2. Cell Mol Neurobiol. 2009. PMID: 19048366 Free PMC article.
-
Ischemic Postconditioning Reduces NMDA Receptor Currents Through the Opening of the Mitochondrial Permeability Transition Pore and KATP Channel in Mouse Neurons.Cell Mol Neurobiol. 2022 May;42(4):1079-1089. doi: 10.1007/s10571-020-00996-y. Epub 2020 Nov 7. Cell Mol Neurobiol. 2022. PMID: 33159622 Free PMC article.
-
Plasticity in the functional properties of NMDA receptors improves network stability during severe energy stress.bioRxiv [Preprint]. 2023 Oct 16:2023.01.19.524811. doi: 10.1101/2023.01.19.524811. bioRxiv. 2023. Update in: J Neurosci. 2024 Feb 28;44(9):e0502232024. doi: 10.1523/JNEUROSCI.0502-23.2024. PMID: 36711958 Free PMC article. Updated. Preprint.
-
Mechanisms of neuroprotection during ischemic preconditioning: lessons from anoxic tolerance.Comp Biochem Physiol A Mol Integr Physiol. 2007 Jun;147(2):291-9. doi: 10.1016/j.cbpa.2006.08.032. Epub 2006 Aug 30. Comp Biochem Physiol A Mol Integr Physiol. 2007. PMID: 17045830 Free PMC article. Review.
References
-
- Akaaboune M, Culican SM, Turney SG, Lichtman JW. Rapid and reversible effects of activity on acetylcholine receptor density at the neuromuscular junction in vivo. Science. 1999;286:503–507. - PubMed
-
- Bickler PE. Reduction of NMDA receptor activity in cerebrocortex of turtles (Chrysemys picta) during 6 wk of anoxia. Am J Physiol. 1998;275:R86–R91. - PubMed
-
- Bickler PE, Buck LT. Adaptations of vertebrate neurons to hypoxia and anoxia: maintaining critical calcium concentrations. J Exp Biol. 1998;201:1141–1152. - PubMed
-
- Bickler PE, Hansen BM. Hypoxia-tolerant neonatal CA1 neurons: relationship of survival to evoked glutamate release and glutamate receptor-mediated calcium changes in hippocampal slices. Dev Brain Res. 1998;106:57–69. - PubMed
-
- Blanton MG, Lo Turco JJ, Kriegstein AR. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989;30:203–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
