Protein phosphatase-1 regulation in the induction of long-term potentiation: heterogeneous molecular mechanisms
- PMID: 10804194
- PMCID: PMC6772695
- DOI: 10.1523/JNEUROSCI.20-10-03537.2000
Protein phosphatase-1 regulation in the induction of long-term potentiation: heterogeneous molecular mechanisms
Abstract
Protein phosphatase inhibitor-1 (I-1) has been proposed as a regulatory element in the signal transduction cascade that couples postsynaptic calcium influx to long-term changes in synaptic strength. We have evaluated this model using mice lacking I-1. Recordings made in slices prepared from mutant animals and also in anesthetized mutant animals indicated that long-term potentiation (LTP) is deficient at perforant path-dentate granule cell synapses. In vitro, this deficit was restricted to synapses of the lateral perforant path. LTP at Schaffer collateral-CA1 pyramidal cell synapses remained normal. Thus, protein phosphatase-1-mediated regulation of NMDA receptor-dependent synaptic plasticity involves heterogeneous molecular mechanisms, in both different dendritic subregions and different neuronal subtypes. Examination of the performance of I-1 mutants in spatial learning tests indicated that intact LTP at lateral perforant path-granule cell synapses is either redundant or is not involved in this form of learning.
Figures
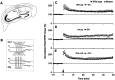

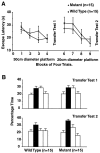
References
-
- Barbas H, Gustafson EL, Greengard P. Comparison of the immunocytochemical localization of DARPP-32 and I-1 in the amygdala and hippocampus of the rhesus monkey. J Comp Neurol. 1993;334:1–18. - PubMed
-
- Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca2+- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. - PubMed
-
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
