The mechanisms of hsp27 antibody-mediated apoptosis in retinal neuronal cells
- PMID: 10804196
- PMCID: PMC6772694
- DOI: 10.1523/JNEUROSCI.20-10-03552.2000
The mechanisms of hsp27 antibody-mediated apoptosis in retinal neuronal cells
Abstract
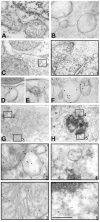
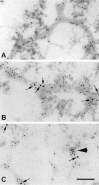
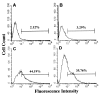
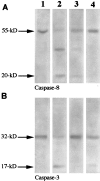
Although elevated titers of serum antibodies to hsp27 accompany human diseases such as cancer and glaucoma, evidence of their pathogenic effects is lacking. Here we present novel evidence that exogenously applied hsp27 antibody enters neuronal cells in human retina by an endocytic mechanism. Subsequent to internalization, hsp27 antibody facilitates apoptotic cell death as characterized by morphological assessment, DNA fragmentation, and the activation of cysteine aspartic acid proteases. In addition, we demonstrate that after internalization, hsp27 antibody is detected in discrete cytoplasmic and nuclear structures and colocalizes to actin cytoskeleton. Hsp27 antibody binding to actin results in depolymerization and proteolytic cleavage of actin in a dose-dependent manner. These results suggest that exogenous hsp27 antibody may induce neuronal apoptosis by inactivating or attenuating the ability of native hsp27 to stabilize actin cytoskeleton, thereby providing a novel mechanism by which autoantibodies to hsp27 may impair cell survival in selective human diseases.
Figures


Similar articles
-
Inhibition of caspase activity in retinal cell apoptosis induced by various stimuli in vitro.Invest Ophthalmol Vis Sci. 1999 Oct;40(11):2660-7. Invest Ophthalmol Vis Sci. 1999. PMID: 10509663
-
Heat shock protein 27 delays Ca2+-induced cell death in a caspase-dependent and -independent manner in rat retinal ganglion cells.Invest Ophthalmol Vis Sci. 2005 Mar;46(3):1085-91. doi: 10.1167/iovs.04-0042. Invest Ophthalmol Vis Sci. 2005. PMID: 15728569
-
Autoantibodies to small heat shock proteins in glaucoma.Invest Ophthalmol Vis Sci. 1998 Nov;39(12):2277-87. Invest Ophthalmol Vis Sci. 1998. PMID: 9804136
-
HSP27 expression regulates CCK-induced changes of the actin cytoskeleton in CHO-CCK-A cells.Am J Physiol. 1999 Dec;277(6):C1032-43. doi: 10.1152/ajpcell.1999.277.6.C1032. Am J Physiol. 1999. PMID: 10600754
-
On the role of Hsp27 in regulating apoptosis.Apoptosis. 2003 Jan;8(1):61-70. doi: 10.1023/a:1021601103096. Apoptosis. 2003. PMID: 12510153 Review.
Cited by
-
Regulation of retinal proteome by topical antiglaucomatous eye drops in an inherited glaucoma rat model.PLoS One. 2012;7(7):e33593. doi: 10.1371/journal.pone.0033593. Epub 2012 Jul 5. PLoS One. 2012. PMID: 22792152 Free PMC article.
-
Autoantibodies against retinal proteins in paraneoplastic and autoimmune retinopathy.BMC Ophthalmol. 2004 Jun 4;4:5. doi: 10.1186/1471-2415-4-5. BMC Ophthalmol. 2004. PMID: 15180904 Free PMC article.
-
Association between open-angle glaucoma and gene polymorphism for heat-shock protein 70-1.Jpn J Ophthalmol. 2007 Nov-Dec;51(6):417-23. doi: 10.1007/s10384-007-0475-9. Epub 2007 Dec 21. Jpn J Ophthalmol. 2007. PMID: 18158591
-
HspB1, HspB5 and HspB4 in Human Cancers: Potent Oncogenic Role of Some of Their Client Proteins.Cancers (Basel). 2014 Feb 7;6(1):333-65. doi: 10.3390/cancers6010333. Cancers (Basel). 2014. PMID: 24514166 Free PMC article.
-
[Autoimmunity and glaucoma].Ophthalmologe. 2019 Jan;116(1):18-27. doi: 10.1007/s00347-018-0658-4. Ophthalmologe. 2019. PMID: 29427020 Review. German.
References
-
- Adamus G, Machnicki M, Seigel GM. Apoptotic retinal cell death induced by autoantibodies of cancer-associated retinopathy. Invest Ophthalmol Vis Sci. 1997;38:283–291. - PubMed
-
- Alarcon-Segovia D, Ruiz-Argüelles A, Fishbein E. Antibody to nuclear ribonucleoprotein penetrates live human mononuclear cells through Fc receptors. Nature. 1978;271:67–69. - PubMed
-
- Alarcon-Segovia D, Ruiz-Argüelles A, Lorente L. Broken dogma: penetration of autoantibodies into living cells. Immunol Today. 1996;17:163–164. - PubMed
-
- Aquino DA, Capello E, Weisstein J, Sanders V, Lopez C, Tourtellotte WW, Brosnan CF, Raine CS, Norton WT. Multiple sclerosis: altered expression of 70- and 27-kDa heat shock proteins in lesions and myelin. J Neuropathol Exp Neurol. 1997;56:664–672. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
