Pregnenolone sulfate modulates inhibitory synaptic transmission by enhancing GABA(A) receptor desensitization
- PMID: 10804198
- PMCID: PMC6772674
- DOI: 10.1523/JNEUROSCI.20-10-03571.2000
Pregnenolone sulfate modulates inhibitory synaptic transmission by enhancing GABA(A) receptor desensitization
Abstract
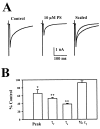
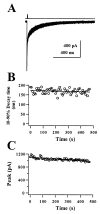
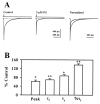
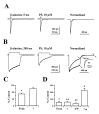
We examined the effects of the neurosteroid pregnenolone sulfate (PS) on GABA(A) receptor-mediated synaptic currents and currents elicited by rapid applications of GABA onto nucleated outside-out patches in cultured postnatal rat hippocampal neurons. At 10 microm, PS significantly depressed peak responses and accelerated the decay of evoked inhibitory synaptic currents. In nucleated outside-out patches, PS depressed peak currents and speeded deactivation after 5 msec applications of a saturating concentration of GABA. PS also increased the rate and degree of macroscopic GABA receptor desensitization during prolonged GABA applications. In a paired GABA application paradigm, PS slowed the rate of recovery from desensitization. In contrast to its prominent effects on currents produced by saturating GABA concentrations, PS had only small effects on peak currents and failed to alter deactivation after brief applications of the weakly desensitizing GABA(A) receptor agonists taurine and beta-alanine. However, when beta-alanine was applied for a sufficient duration to promote receptor desensitization, PS augmented macroscopic desensitization and slowed deactivation. These results suggest that PS inhibits GABA-gated chloride currents by enhancing receptor desensitization and stabilizing desensitized states. This contention is supported by kinetic modeling studies in which increases in the rate of entry into doubly liganded desensitized states mimic most effects of PS.
Figures




Similar articles
-
Lanthanum-mediated modification of GABAA receptor deactivation, desensitization and inhibitory synaptic currents in rat cerebellar neurons.J Physiol. 1998 Sep 15;511 ( Pt 3)(Pt 3):647-61. doi: 10.1111/j.1469-7793.1998.647bg.x. J Physiol. 1998. PMID: 9714849 Free PMC article.
-
Zinc inhibits miniature GABAergic currents by allosteric modulation of GABAA receptor gating.J Neurosci. 2000 Dec 1;20(23):8618-27. doi: 10.1523/JNEUROSCI.20-23-08618.2000. J Neurosci. 2000. PMID: 11102466 Free PMC article.
-
Neurosteroid prolongs GABAA channel deactivation by altering kinetics of desensitized states.J Neurosci. 1997 Jun 1;17(11):4022-31. doi: 10.1523/JNEUROSCI.17-11-04022.1997. J Neurosci. 1997. PMID: 9151718 Free PMC article.
-
Molecular and Regulatory Mechanisms of Desensitization and Resensitization of GABAA Receptors with a Special Reference to Propofol/Barbiturate.Int J Mol Sci. 2020 Jan 15;21(2):563. doi: 10.3390/ijms21020563. Int J Mol Sci. 2020. PMID: 31952324 Free PMC article. Review.
-
M currents.Ion Channels. 1988;1:55-94. doi: 10.1007/978-1-4615-7302-9_2. Ion Channels. 1988. PMID: 3155026 Review. No abstract available.
Cited by
-
Inhibition of the GABAA Receptor by Sulfated Neurosteroids: A Mechanistic Comparison Study between Pregnenolone Sulfate and Dehydroepiandrosterone Sulfate.J Mol Neurosci. 2015 Aug;56(4):868-877. doi: 10.1007/s12031-015-0527-4. Epub 2015 Mar 1. J Mol Neurosci. 2015. PMID: 25725785
-
Endogenous neuro-protectants in ammonia toxicity in the central nervous system: facts and hypotheses.Metab Brain Dis. 2005 Dec;20(4):253-63. doi: 10.1007/s11011-005-7904-6. Metab Brain Dis. 2005. PMID: 16382336 Review.
-
The insecticide fipronil and its metabolite fipronil sulphone inhibit the rat alpha1beta2gamma2L GABA(A) receptor.Br J Pharmacol. 2008 Nov;155(5):783-94. doi: 10.1038/bjp.2008.309. Epub 2008 Jul 28. Br J Pharmacol. 2008. PMID: 18660823 Free PMC article.
-
Is connexin36 critical for GABAergic hypersynchronization in the hippocampus?J Physiol. 2011 Apr 1;589(Pt 7):1663-80. doi: 10.1113/jphysiol.2010.201491. Epub 2011 Feb 7. J Physiol. 2011. PMID: 21300748 Free PMC article.
-
Activation-dependent properties of pregnenolone sulfate inhibition of GABAA receptor-mediated current.J Physiol. 2003 Aug 1;550(Pt 3):679-91. doi: 10.1113/jphysiol.2003.043810. J Physiol. 2003. PMID: 12938673 Free PMC article.
References
-
- Baulieu E-E, Robel P. Neurosteroids: a new brain function? J Steroid Biochem Mol Biol. 1990;37:395–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
