Vasoactive intestinal peptide and pituitary adenylyl cyclase-activating polypeptide inhibit tumor necrosis factor-alpha production in injured spinal cord and in activated microglia via a cAMP-dependent pathway
- PMID: 10804204
- PMCID: PMC6772690
- DOI: 10.1523/JNEUROSCI.20-10-03622.2000
Vasoactive intestinal peptide and pituitary adenylyl cyclase-activating polypeptide inhibit tumor necrosis factor-alpha production in injured spinal cord and in activated microglia via a cAMP-dependent pathway
Abstract
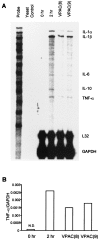
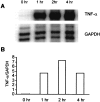
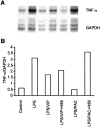
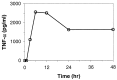
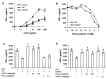
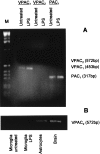
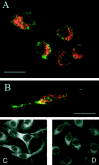
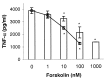
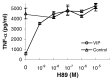
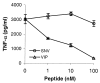
Tumor necrosis factor-alpha (TNF-alpha) production accompanies CNS insults of all kinds. Because the neuropeptide vasoactive intestinal peptide (VIP) and the structurally related peptide pituitary adenylyl cyclase-activating polypeptide (PACAP) have potent anti-inflammatory effects in the periphery, we investigated whether these effects extend to the CNS. TNF-alpha mRNA was induced within 2 hr after rat spinal cord transection, and its upregulation was suppressed by a synthetic VIP receptor agonist. Cultured rat microglia were used to examine the mechanisms underlying this inhibition because microglia are the likely source of TNF-alpha in injured CNS. In culture, increases in TNF-alpha mRNA resulting from lipopolysaccharide (LPS) stimulation were reduced significantly by 10(-7) m VIP and completely eliminated by PACAP at the same concentration. TNF-alpha protein levels were reduced 90% by VIP or PACAP at 10(-7) m. An antagonist of VPAC(1) receptors blocked the action of VIP and PACAP, and a PAC(1) antagonist blocked the action of PACAP. A direct demonstration of VIP binding on microglia and the existence of mRNAs for VPAC(1) and PAC(1) (but not VPAC(2)) receptors argue for a receptor-mediated effect. The action of VIP is cAMP-mediated because (1) activation of cAMP by forskolin mimics the action; (2) PKA inhibition by H89 reverses the neuropeptide-induced inhibition; and (3) the lipophilic neuropeptide mimic, stearyl-norleucine(17) VIP (SNV), which does not use a cAMP-mediated pathway, fails to duplicate the inhibition. We conclude that VIP and PACAP inhibit the production of TNF-alpha from activated microglia by a cAMP-dependent pathway.
Figures
Similar articles
-
Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit chemokine production in activated microglia.Glia. 2002 Aug;39(2):148-61. doi: 10.1002/glia.10098. Glia. 2002. PMID: 12112366
-
Pituitary adenylate cyclase-activating peptide stimulates acute progesterone production in rat granulosa/Lutein cells via two receptor subtypes.Biol Reprod. 2000 Jul;63(1):206-12. doi: 10.1095/biolreprod63.1.206. Biol Reprod. 2000. PMID: 10859261
-
Regulation of the rat proopiomelanocortin gene expression in AtT-20 cells. II: Effects of the pituitary adenylate cyclase-activating polypeptide and vasoactive intestinal polypeptide.Endocrinology. 1997 May;138(5):1930-4. doi: 10.1210/endo.138.5.5116. Endocrinology. 1997. PMID: 9112389
-
Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions.Pharmacol Rev. 2000 Jun;52(2):269-324. Pharmacol Rev. 2000. PMID: 10835102 Review.
-
The role of vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating polypeptide in the neural pathways controlling the lower urinary tract.J Mol Neurosci. 2008 Nov;36(1-3):227-40. doi: 10.1007/s12031-008-9090-6. Epub 2008 Aug 2. J Mol Neurosci. 2008. PMID: 18677446 Free PMC article. Review.
Cited by
-
VIP plasma levels associate with survival in severe COVID-19 patients, correlating with protective effects in SARS-CoV-2-infected cells.J Leukoc Biol. 2022 May;111(5):1107-1121. doi: 10.1002/JLB.5COVA1121-626R. Epub 2022 Mar 24. J Leukoc Biol. 2022. PMID: 35322471 Free PMC article.
-
A Synthetic Agonist to Vasoactive Intestinal Peptide Receptor-2 Induces Regulatory T Cell Neuroprotective Activities in Models of Parkinson's Disease.Front Cell Neurosci. 2019 Sep 18;13:421. doi: 10.3389/fncel.2019.00421. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31619964 Free PMC article.
-
Use of laser microdissection in the investigation of facial motoneuron and neuropil molecular phenotypes after peripheral axotomy.Exp Neurol. 2010 Sep;225(1):94-103. doi: 10.1016/j.expneurol.2010.05.019. Epub 2010 Jun 4. Exp Neurol. 2010. PMID: 20570589 Free PMC article.
-
Vasoactive intestinal peptide: a neuropeptide with pleiotropic immune functions.Amino Acids. 2013 Jul;45(1):25-39. doi: 10.1007/s00726-011-1184-8. Epub 2011 Dec 3. Amino Acids. 2013. PMID: 22139413 Free PMC article. Review.
-
Macrophage Resistance to HIV-1 Infection Is Enhanced by the Neuropeptides VIP and PACAP.PLoS One. 2013 Jun 20;8(6):e67701. doi: 10.1371/journal.pone.0067701. Print 2013. PLoS One. 2013. PMID: 23818986 Free PMC article.
References
-
- Akassoglou K, Bauer J, Kassiotis G, Pasparakis M, Lassmann H, Kollias G, Probert L. Oligodendrocyte apoptosis and primary demyelination induced by local TNF/p55TNF receptor signaling in the central nervous system of transgenic mice: models for multiple sclerosis with primary oligodendrogliopathy. Am J Pathol. 1998;153:801–813. - PMC - PubMed
-
- Albrecht H, Schook LB, Jongeneel CV. Nuclear migration of NF-κB correlates with TNF-α mRNA accumulation. J Inflamm. 1995;45:64–71. - PubMed
-
- Barger SW, Horster D, Furukawa K, Goodman Y, Krieglstein J, Mattson MP. Tumor necrosis factors α and β protect neurons against amyloid β-peptide toxicity: evidence for involvement of a κB-binding factor and attenuation of peroxide and Ca2+ accumulation. Proc Natl Acad Sci USA. 1995;92:9328–9332. - PMC - PubMed
-
- Bartholdi D, Schwab ME. Expression of pro-inflammatory cytokine and chemokine mRNA upon experimental spinal cord injury in mouse: an in situ hybridization study. Eur J Neurosci. 1997;9:1422–1438. - PubMed
-
- Boudard F, Bastide M. Inhibition of mouse T-cell proliferation by CGRP and VIP: effects of these neuropeptides on IL-2 production and cAMP synthesis. J Neurosci Res. 1991;29:29–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
