N-CAM binding inhibits the proliferation of hippocampal progenitor cells and promotes their differentiation to a neuronal phenotype
- PMID: 10804205
- PMCID: PMC6772667
- DOI: 10.1523/JNEUROSCI.20-10-03631.2000
N-CAM binding inhibits the proliferation of hippocampal progenitor cells and promotes their differentiation to a neuronal phenotype
Abstract
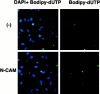
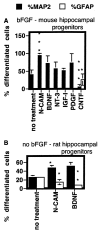
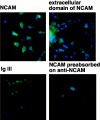
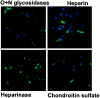
Cell adhesion molecules (CAMs) play important roles during the development of the nervous system. On the basis of our previous observations that binding of the neural CAM (N-CAM) inhibits astrocyte proliferation and alters gene expression, we hypothesized that N-CAM may influence the balance between the proliferation and the differentiation of neural progenitor cells. Rat and mouse hippocampal progenitor cells were cultured and showed dependence on basic FGF for proliferation, immunoreactivity for nestin, the presence of limited numbers of differentiated cells, and the ability to generate glial cells and neurons under different culture conditions. Addition of soluble N-CAM reduced cell proliferation in a dose-dependent manner with no evidence of apoptosis. The inhibition of proliferation by N-CAM was accompanied by an induction of differentiation to the neuronal lineage, as indicated by a twofold increase in the percentage of microtubule-associated protein 2-positive cells even in the presence of mitogenic growth factors. Experiments using hippocampal cells from N-CAM knock-out mice indicated that N-CAM on the cell surface is not required for these effects, suggesting the existence of heterophilic signaling. These results support a role for N-CAM and N-CAM ligands in the inhibition of proliferation and the induction of neural differentiation of hippocampal neural progenitor cells.
Figures





References
-
- Azizeh BY, Cribbs DH, Kreng VM, Cotman CW. Cross-linking of N-CAM receptors on neurons induces programmed cell death. Brain Res. 1998;796:20–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
